A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL-II|20 VideosGASEOUS STATE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL-II COMPREHENSION-I|5 VideosGASEOUS STATE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL-2|9 VideosENVIRONMENTAL CHEMISTRY
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL -II (MULTIPLE CORRECT QUESTIONS)|20 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|14 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-GASEOUS STATE-ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL-I
- A glass bulb is coninected to an open limb manometer. The level of mer...
Text Solution
|
- The value of van der Waals constant 'a' is makimum for
Text Solution
|
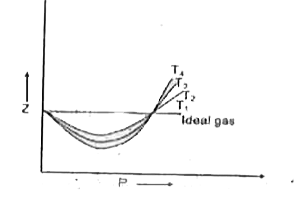
- Compressibility factor (Z) is plotted against pressure at different te...
Text Solution
|
- A sealed tube which can withstand a pressure of 3 atm sphere is filled...
Text Solution
|
- For non-zero value of force of attraction between gas molecules gas eq...
Text Solution
|
- Distribution of molecules with velocity is represented by the curve. P...
Text Solution
|
- According to Charle's law
Text Solution
|
- For a given mass of gas, if pressure is reduced to half and temperatur...
Text Solution
|
- The density of O(2) gas at 25^(@)C is 1.458 mg/lt at one atm pressure....
Text Solution
|
- I, II, III are three isothem respectively at T(1), T(2) & T(3) tempera...
Text Solution
|
- NH(3) is liquefied more easily than N(2) Hence.
Text Solution
|
- 0.2 mole sample of hydrocarbon C(x)H(y) yields after complete combusti...
Text Solution
|
- When 2 gm A gas is introduced into an evacuated flask kept at 25^(@)C,...
Text Solution
|
- Air open vessel at 127^(@)C is heated until 1//5^(th) of air in it has...
Text Solution
|
- Which of the following volume (V) temperature (T) plots represents the...
Text Solution
|
- A gas cylinder containing cooking gas can withstand a pressure of 14.9...
Text Solution
|
- 0.5 mole each of H(2), SO(2) & CH(4) are kept in container. A hole was...
Text Solution
|
- A 2.24 litre cylinder of oxygen at NTP is found to develop a leakage. ...
Text Solution
|
- Two glass bulbs A and B are connected by very small tube having a stop...
Text Solution
|
- In a closed vessel, a gas is heated from 300 K to 600 K the kinetic en...
Text Solution
|