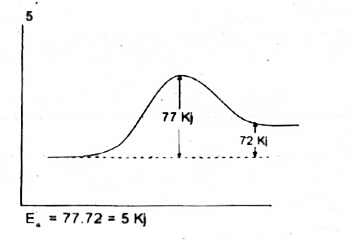
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS AND RADIOACTIVITY
FIITJEE|Exercise Assignment problems (Subjective) numerical based Level-1|5 VideosCHEMICAL KINETICS AND RADIOACTIVITY
FIITJEE|Exercise Assignment problems (Subjective) Level-II|15 VideosCHEMICAL KINETICS AND RADIOACTIVITY
FIITJEE|Exercise Matrix Match|2 VideosCHEMICAL EQUILIBRIUM
FIITJEE|Exercise MATCHING TYPE QUESTIONS|3 VideosCHEMISTRY IN EVERY DAY LIFE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II (Numerical Based Questions)|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-CHEMICAL KINETICS AND RADIOACTIVITY-Single integer type question
- There is formation of H(2)O(2) in the upper atmosphere. H(2)O+O to 2...
Text Solution
|
- If in the fementation of sugar in an enzymatic solution thatis 0.12m t...
Text Solution
|
- In a reaction , the time required to complete half of the reaction was...
Text Solution
|
- If the t(1//2) for a first order reaction is 0.4 min, the time for 99....
Text Solution
|
- An unstable isotope of carbon has (N)/(P) ratio of 1.33 times greater ...
Text Solution
|
- What would be the atomic number of the missing species in the given tr...
Text Solution
|
- For a second order reaction, t(75%)=x t(50%) Find the value of x.
Text Solution
|
- The first order gaseous decomposition of N(2)O(4) in to NO(2) has a ra...
Text Solution
|
- If lactobacillus bacteria (which converts milk to curd) multiply in mi...
Text Solution
|
- The velocity of a reaction is tripled for every 10^(@)C rise in temper...
Text Solution
|
- The decomposition of ethane into ethene and hydrogen involves 5 differ...
Text Solution
|