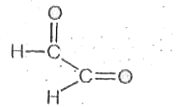
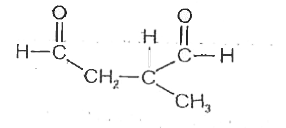
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - C) (Previous Years Questions)|69 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - A) (Obejctive type question)|55 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HYDROCARBONS-Assignment(Section - B) (Obejctive type question)
- CH(3)-CH=CH-CHO overset(A)to CH(3)-CH=CH-CH(3) The best suitable rea...
Text Solution
|
- The most suitable reagent for given conversion is
Text Solution
|
- Choose the corrrect option
Text Solution
|
- In the above reaction product (B) is
Text Solution
|
- The product will be
Text Solution
|
- Product C is
Text Solution
|
- Natural rubber is a polymer of isoprene(2-methylbuta-1, 3-diene). If n...
Text Solution
|
- Product C is
Text Solution
|
- Dehydration by conc. H(2)SO(4) is the most difficult in
Text Solution
|
- Which of the folllwing can be form methane gas with methyl magnesium b...
Text Solution
|
- Which of the following compound is paramagnetic ?
Text Solution
|
- CaC(2) overset(H(2)O)to A overset(Cu(2)Cl(2))underset(NH(4)Cl)to B ove...
Text Solution
|
- In which of the following dehydration by conc. H(2)SO(4) no rearrangem...
Text Solution
|