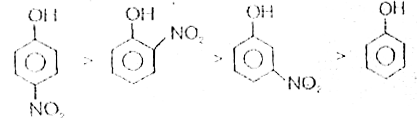
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Exercise|40 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Assignment Section - A (Objective type questions)|45 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Assignment Section -D (Assertion - reason type question)|19 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Try Yourself|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE- ALCOHOLS, PHENOLS AND ETHERS-Try yourself
- Arrange the following in increasing order of their (i) Solubility...
Text Solution
|
- (a) Why acidic nature of alcohol and phenol increases with electron ...
Text Solution
|
- For the preparation of anisold which one is preferable reaction and w...
Text Solution
|
- Predict final products in the reactions
Text Solution
|