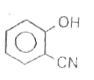
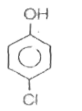
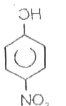
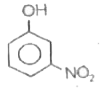
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Assignment Section - A (Objective type questions)|45 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Assignment Section - B (Objective type questions)|24 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Try yourself|4 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Try Yourself|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE- ALCOHOLS, PHENOLS AND ETHERS-Exercise
- Carbolic acid is
Text Solution
|
- Which of the following is a trihydric phenol ?
Text Solution
|
- Which of the following is the strongest acid ?
Text Solution
|
- Electrophilic subsitution reaction in phenol take place at :
Text Solution
|
- The following reaction is known as : Phenol overset(1.CHCl(3)//NaO...
Text Solution
|
- What amount of bromine will be required to convert 2g of phenol into 2...
Text Solution
|
- Identify the product Z in the following sequence of reactions "pheno...
Text Solution
|
- Phenol can be distinguished from ethanol by the following reagents exc...
Text Solution
|
- The most suitable convenient method of separation of o-and p-nitrophen...
Text Solution
|
- Neutral FeCl(3) gives purple colour with
Text Solution
|
- Asqirin is obtained by the reaction of salicylic acid with
Text Solution
|
- Zine powder . In the above reaction X will be
Text Solution
|
- The ionization constant of a phenol is higher than that of ethanol bec...
Text Solution
|
- Can be
Text Solution
|
- The number of metamers possible for C(4)H(10) O is
Text Solution
|
- Which of the following has maximum bond angle around oxygen ?
Text Solution
|
- OCH(3) group is
Text Solution
|
- CH(3) CH(2) +cl+Ag(2)O overset(Delta)to Product . Product formed in ...
Text Solution
|
- Product . Product of the reaction is
Text Solution
|
- The ether that undergoes electrophilic substitution reactions is
Text Solution
|