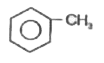
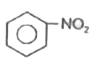
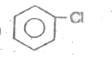
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following undergoes nitration most easily?
Text Solution
|
- Which of the following undergoes Hydrolysis most easily:
Text Solution
|
- Which of the following undergoes Hydrolysis most easily:
Text Solution
|
- Out of benzene, m–dinitrobenzene and toluene which will undergo nitrat...
Text Solution
|
- Which of the following compound undergo hydrolysis most easily :
Text Solution
|
- Which one of the following undergoes nitration most readily ?
Text Solution
|
- Out of benzene, m-dinitrobenzene and toluene which will undergo nitrat...
Text Solution
|
- Out of benzen m - dinitrobenzene and toluene which will undergo nitra...
Text Solution
|
- Out of benzene, m–dinitrobenzene and toluene which will undergo nitrat...
Text Solution
|