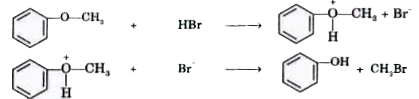Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise NCERT (IN-TEXT EXERCISES)|12 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise NCERT (TEXTBOOK EXERCISES)|33 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise CONCEPTUAL QUESTIONS 1|26 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS-CONCEPTUAL QUESTIONS 2
- Write structure of phenyl isopentyl ether. Give its IUPAC name.
Text Solution
|
- Anisole on reaction with HI gives phenol and CH(3)-I as the main produ...
Text Solution
|
- Ethers are relatively inert. Justify.
Text Solution
|
- Why di tert-butyl ether cannot be prepared by Williamson synthesis ?
Text Solution
|
- Name the pair of alkyl halide and alkoxide for the preparation of ethy...
Text Solution
|
- What products are obtained when.
Text Solution
|
- HI is a better reagent than HBr for cleavage of ether. Explain.
Text Solution
|
- Why are the boiling points of ethers lower than those of isomeric alco...
Text Solution
|
- Explain why cleavage of phenyl alkyl others with HBr always produces p...
Text Solution
|
- An ether possesses dipole moment even if the alkyl groups present in i...
Text Solution
|
- Why a non-symmetrical ether is not prepared by heating a mixture of RO...
Text Solution
|
- How do you account for the miscibility of ethoxyethane with water ?
Text Solution
|
- Butan-1-ol has higher boiling point than diethyl ether. Assign reason.
Text Solution
|
- (CH(3))(3)C-OCH(3) on reaction with HI gives (CH(3))(3)C-I and CH(3)-O...
Text Solution
|
- Complete the reaction :
Text Solution
|