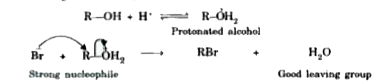Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (MULTIPLE CHOICE QUESTIONS) (M.C.Q) (MULTIPLE CHOICE QUESTIONS with only one corrrect answer)|30 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (MULTIPLE CHOICE QUESTIONS) (from competitive examinations) (AIPMT, NEET & OTHER STATE BOARD.S MEDICAL ENTRANCE)|23 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise Revision Exercises (Long Answer Questions)|2 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS-HIGHER ORDER THINKING SKILLS (ADVANCED LEVEL) (QUESTIONS WITH ANSWERS)
- Acid catalysed dehydration of t-butanol is faster than that of n-butan...
Text Solution
|
- Hydration of 3-phenyl-1.butene with dil. H(2)SO(4), is not a satisfact...
Text Solution
|
- Give the product and show the steps in (i) the hydration of cyclobutyl...
Text Solution
|
- Arrange the following alcohols in the increasing order of reactivity w...
Text Solution
|
- Show steps for the conversion of ethene to divinyl ether.
Text Solution
|
- Cyclobutyl bromide on treatment with magnesium in dry ether forms an o...
Text Solution
|
- Dehydration of alcohol to form an alkene is always carried out with co...
Text Solution
|
- Alcohols donot react with NaBr but when H.SO, is added they form alkyl...
Text Solution
|
- Cyclic C(4)H(7)OH has five isomers. Write their structure and names.
Text Solution
|
- Neopentyl alcohol reacts with concentrated HBr to give 2-bromo-2-methy...
Text Solution
|
- An ether, (A) having molecular formula, C(6)H(14)O, when treated with ...
Text Solution
|
- An organic compound A(C(2)H(6)O) reacts with sodium to form a compound...
Text Solution
|
- A compound D(C(6) H(10)O) upon treatment with alkaline solution of iod...
Text Solution
|