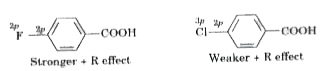Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise COMPETITION FILE ( OBJECTIVE TYPE QUESTIONS - MULTIPLE CHOICE QUESTIONS (MCQ) ( A. MULTIPLE CHOICE QUESTIONS )|35 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise COMPETITION FILE ( OBJECTIVE TYPE QUESTIONS - MULTIPLE CHOICE QUESTIONS (MCQ) ( B. MULTIPLE CHOICE QUESTIONS )|86 VideosALDEHYDES ,KETONES AND CARBOXYLIC ACIDS
MODERN PUBLICATION|Exercise REVISION EXERCISES (OBJECTIVE QUESTIONS - LONG ANSWER QUESTIONS )|8 VideosALCOHOLS, PHENOLS AND ETHERS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST (FOR BOARD EXAMINATION)|12 VideosBIOMOLECULES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST FOR BOARD EXAMINATION|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ALDEHYDES ,KETONES AND CARBOXYLIC ACIDS-HOTS HIGHER ORDER THINKING SKILLS
- Hydrogen of acetadehyde are not highly acidic medium explain .
Text Solution
|
- Oximes are more acidic than hydroxylamine. Explain.
Text Solution
|
- Di-tert-butyl ketone does not give precipitate with NaHSO4 whereas ace...
Text Solution
|
- Dialkyl cadmium is used to prepare ketones from acid chlorides and no...
Text Solution
|
- What is the function of Rochelle salt in Fehling's solution ?
Text Solution
|
- Aldehydes usually donot form stable hydrates but chloral normally exis...
Text Solution
|
- Treatment of C6 H5 CHO with HCN gives a mixture of two isomers which c...
Text Solution
|
- A ketone A, which undergoes haloform reaction, gives compound B on red...
Text Solution
|
- An organic compound A(C3 H6 O) is resistant to oxidation but forms com...
Text Solution
|
- An organic compound (A) (C(6)H(10)O) on reaction with CH(3)MgBr follow...
Text Solution
|
- Identify (x) and (Y) in the following reaction sequence :
Text Solution
|
- Explain the fact that the C-O bond length in RCOOH is shorter than in ...
Text Solution
|
- Although p-hydroxy benzoic acid is less acidic than benzoic acid, orth...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- Addition of Grignard reagents to dry ice followed by hydrolysis gives ...
Text Solution
|