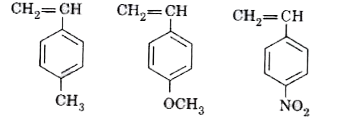
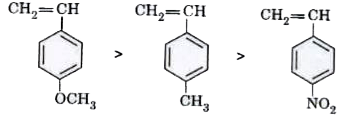
Text Solution
Verified by Experts
Topper's Solved these Questions
POLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (A. MULTIPLE CHOICE QUESTIONS)(Polymers and their Classifications)|10 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (A. MULTIPLE CHOICE QUESTIONS)(Some Important Polymers)|15 VideosPOLYMERS
MODERN PUBLICATION|Exercise Revision Exercises (Objective Questions)(Short Answer Questions)|46 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|5 VideosSOLID STATE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-POLYMERS-HIGHER ORDER THINKING SKILLS (ADVANCED LEVEL)
- Why should one always use purest monomer in free radical polymerisatio...
Text Solution
|
- Will you prefer to polymerize acrylonitrile under anionic or cationic ...
Text Solution
|
- Can a co-polymer be formed both in additin and condensation polymerisa...
Text Solution
|
- What is the monomer of (--CH(2)-CH(2)-O-CH(2)-CH(2)-O--)(n)?
Text Solution
|
- Arrange the following alkenes towards order of increasing reactivity i...
Text Solution
|
- Arrange the following alkenes in order of increasing reactivity toward...
Text Solution
|
- Poly (butylenes terephthalate) is a plastic material used in automotiv...
Text Solution
|
- Arrange the following groups of monomers in order of decreasing abilit...
Text Solution
|
- Depict a free radical mode of addition polymerisation in isoprene.
Text Solution
|
- Kodel polyester is formed by trans-esterification of dimethyl terephth...
Text Solution
|