Text Solution
Verified by Experts
Topper's Solved these Questions
NUCLEI
NEW JOYTHI PUBLICATION|Exercise CONTINUOUS EVALUATION|2 VideosNUCLEI
NEW JOYTHI PUBLICATION|Exercise CONTINUOUS EVALUATION(ASSIGNMENT)|9 VideosNUCLEI
NEW JOYTHI PUBLICATION|Exercise PRACTICE PROBLEMS FOR SELF ASSESSMENT|18 VideosMOVING CHARGES AND MAGNETISM
NEW JOYTHI PUBLICATION|Exercise COMPETITIVE EXAM CORNER|28 VideosRAY OPTICS AND OPTICAL INSTRUMENTS
NEW JOYTHI PUBLICATION|Exercise COMPETITIVE EXAM CORNER|26 Videos
Similar Questions
Explore conceptually related problems
NEW JOYTHI PUBLICATION-NUCLEI-EVALUATION QUESTIONS AND ANSWERS
- There are two nuclides ""(10)^(22)Ne and ""(11)^(22)Na. a. What are...
Text Solution
|
- What are Isomers?Give examples.
Text Solution
|
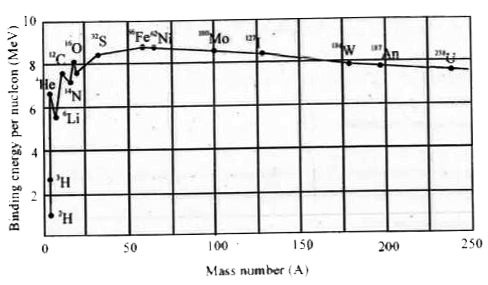
- a. What does the graph represent ? b.What is the importance of this...
Text Solution
|
- In certain isobars the number of protons of one isobar is equal to the...
Text Solution
|
- Z,A and M represent the atomic number, mass number and rest mass of a ...
Text Solution
|
- ""(Z)^(A)X to ""(Z + 1)^(A)Y + ""(-1)^(0)e + barupsilon + "energy" a...
Text Solution
|
- A nuclear event is shown in the diagram. a. Name the nuclear phen...
Text Solution
|
- The radioacitve decay of an element is shown in the graph. a. What i...
Text Solution
|
- Mention the different units of radioactivity.
Text Solution
|
- Why are neutrons very effective as bombarding particles?
Text Solution
|
- ""(72)^(180)A overset(a)(rarr)B overset(beta)(rarr)C overset(alpha)(ra...
Text Solution
|
- From the above table we can see that the ratio of their atomic masses ...
Text Solution
|
- a. What is positron ? " " b. What is its charge ?
Text Solution
|
- What is " " i. neutrino " " ii. antineutrino? Explain with exa...
Text Solution
|
- alpha-particles have a high ionizing power. Justify your answer.
Text Solution
|
- a. Are the equations of nuclear reactions such as ""(7)^(14)N + ""(2)^...
Text Solution
|
- Complete the nuclear reaction a. ""(5)^(10)B + ………… to ""3^7Li + ""(...
Text Solution
|
- a. Which is more stable , ""(3)^(7)Li " or " ""(3)^(4)Li ? b. Give...
Text Solution
|
- What is meant by chain reaction ?
Text Solution
|
- a. Are heavy nuclei stable or unstable ? b. Give reason.
Text Solution
|