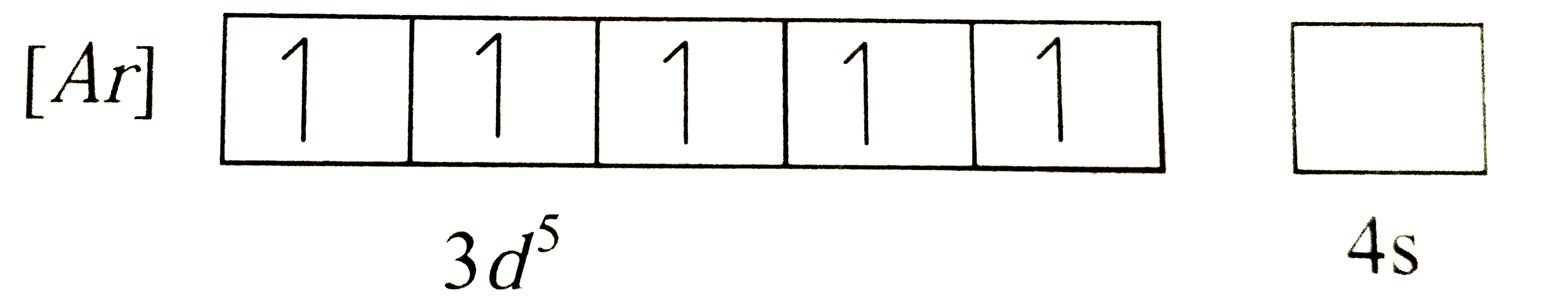A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS -ATOMIC STRUCTURE-Quantum numbers , Electronic configuration and Shape of orbitals
- Which orbital give an electron the greatest probability of being found...
Text Solution
|
- The electronic configuration of chromium (z=24) is
Text Solution
|
- Which of the following metal ions will have maximum number of unpaired...
Text Solution
|
- The correct set of four quantum numbers for outermost electron of pota...
Text Solution
|
- The maximum number of electrons accommodated in 5f orbitals are
Text Solution
|
- All electrons on the 4p sub-shell must be characterized by the quantum...
Text Solution
|
- The maximum number of electrons in an atom with l=2 and n=3 is
Text Solution
|
- Which of the following sets of quantum number is not possible for an e...
Text Solution
|
- The valency of the element having atomic number 9 is
Text Solution
|
- The most stable orbitals are
Text Solution
|
- Which of the following sets of quantum number is restricted
Text Solution
|
- In a given atom no two electrons can have the same values for all the ...
Text Solution
|
- Which one is the electronic configuration of Fe^(+2)
Text Solution
|
- According to Aufbau's principle, which of the three 4d, 5p and 5s will...
Text Solution
|
- For the n=2 energy level, how many orbitals of all kinds are possible
Text Solution
|
- When the principal quantum number (n=3) , the possible values of azimu...
Text Solution
|
- If n + l=6 , then total possible number of subshells would be
Text Solution
|
- Which of the following orbitals are not possible ? 2s, 2p, 3f, 3d.
Text Solution
|
- For sodium atom the number of electrons with m=0 will be
Text Solution
|
- The explanation for the presence of three unpaired electrons in the ni...
Text Solution
|