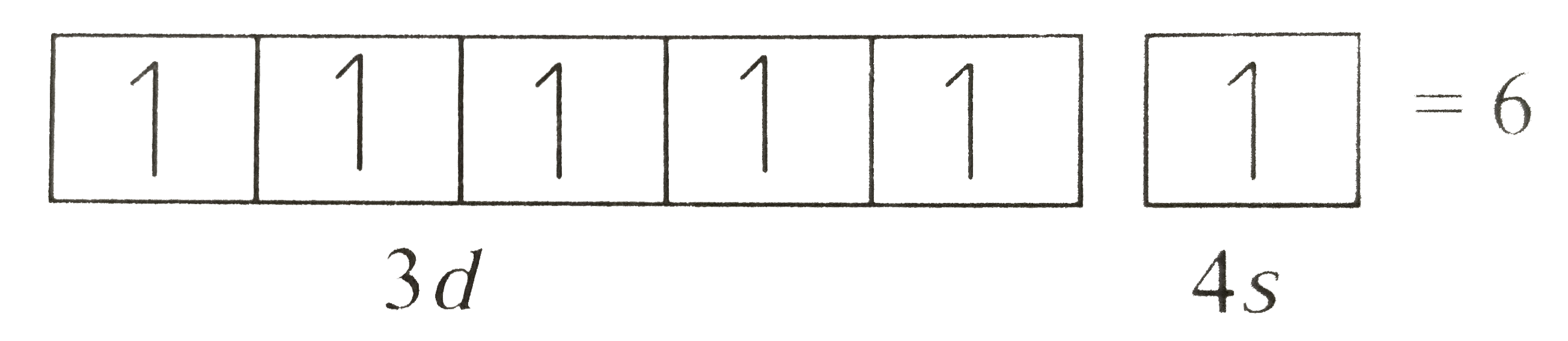
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS -ATOMIC STRUCTURE-Quantum numbers , Electronic configuration and Shape of orbitals
- The explanation for the presence of three unpaired electrons in the ni...
Text Solution
|
- Electron enters the sub-shell for which (n+l) value is minimum. This i...
Text Solution
|
- Following Hund's rule which element contains six unpaired electron
Text Solution
|
- Which of the following sets of quantum numbers is correct for an elect...
Text Solution
|
- Consider the ground state Cr atom (Z = 24). The number of electron w...
Text Solution
|
- In a malti-electrons atom which of the following orbitals deseribed...
Text Solution
|
- Which of the following sets of quantum numbers represents the highest ...
Text Solution
|
- The electrons identified by quantum numbers n and l
Text Solution
|
- Which of the following configuration is correct for iron
Text Solution
|
- The number of orbitals in d sub-shell is
Text Solution
|
- The number of valence electrons in carbon atom is
Text Solution
|
- Pauli's exclusion principle states that
Text Solution
|
- For d subshell , the azimuthal quantum number is
Text Solution
|
- The number of unpaired electrons in carbon atom is -
Text Solution
|
- A completely filled d-orbital (d^10) is
Text Solution
|
- The two electrons in K sub-shell will differ in
Text Solution
|
- Which of the following represents the correct sets of the four quantum...
Text Solution
|
- Which of the following statements is not correct for an electron that ...
Text Solution
|
- The number of d electrons in Fe^(2+) (atomic number of Fe = 26) is not...
Text Solution
|
- Which set of quantum numbers for an electron of an atom is not possibl...
Text Solution
|