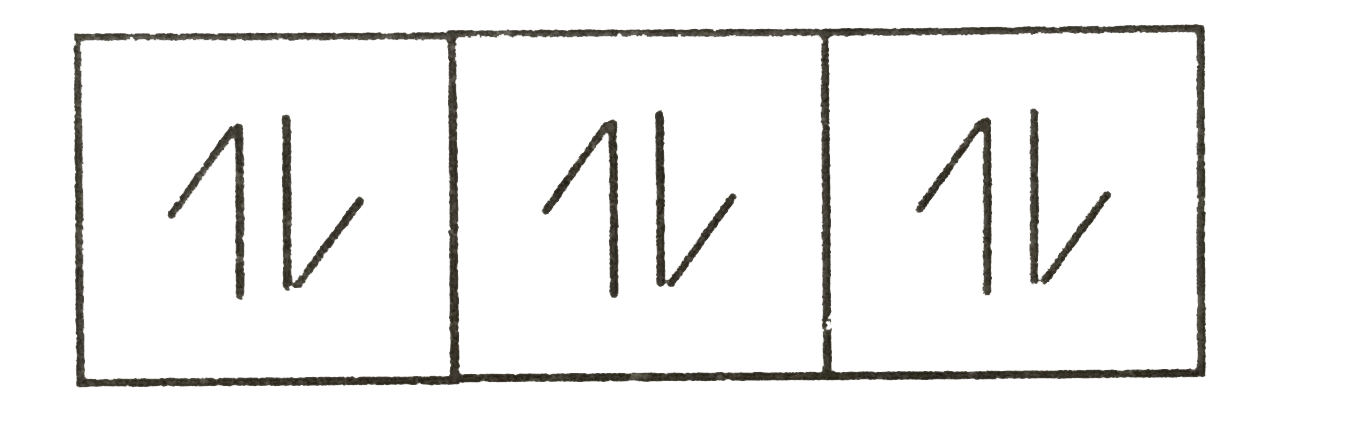
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS -ATOMIC STRUCTURE-Quantum numbers , Electronic configuration and Shape of orbitals
- Identify the CORRECT statement
Text Solution
|
- Which of the following statement(s) is (are) correct
Text Solution
|
- The maximum number of 2p electrons with the electronic spin=-1/2 are
Text Solution
|
- An electron having the quantum numbers n=4, l=3 , m=0 , s=-1/2 would b...
Text Solution
|
- Which of the following sets of quantum numbers is not allowed
Text Solution
|
- The total magnetic quantum numbers for d-orbitals is given by
Text Solution
|
- For n=2 the correct set of azimuthal and magnetic quantum numbers are
Text Solution
|
- An element forms diatomic molecule with a triple bond. The configurati...
Text Solution
|
- Which law represents the pairing of electron in a sub-shell after each...
Text Solution
|
- The ground state electronic configuration of chromium is against
Text Solution
|
- Number of two electron can have the same values of … quantum numbers
Text Solution
|
- The number of orbitals present in the shell with n=4 is
Text Solution
|
- The structure of external most shell of inert gases in
Text Solution
|
- https://d10lpgp6xz60nq.cloudfront.net/physicsimages/PSMATHVIIC08E04016...
Text Solution
|
- Which one pair of atoms or ions will have same configuration ?
Text Solution
|
- Number of nodal centres for 2s orbital
Text Solution
|
- Which of the following sets is possible for quantum numbers
Text Solution
|
- What is the maximum number of electrons that can be associated with a ...
Text Solution
|
- Two electrons occupying the same orbital are distinguished by :
Text Solution
|
- Which of the following pairs of d-orbitals will hare electron density ...
Text Solution
|