Similar Questions
Explore conceptually related problems
Recommended Questions
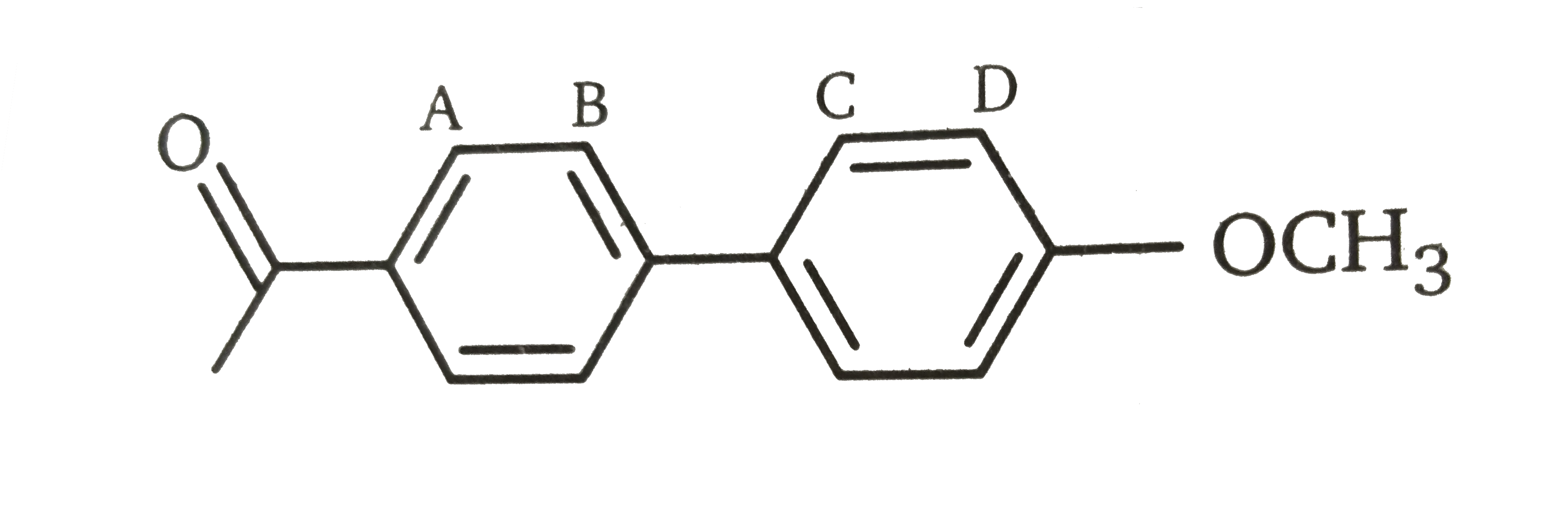
- Which position will be attacked most rapidly by the nitronium ion (-NO...
Text Solution
|
- Attacking or reactive or electrophilic species in nitration of benzene...
Text Solution
|
- Assertion : A mixture of HNO(3) and H(2)SO(4) is used for the nitratio...
Text Solution
|
- Which of the following compounds is the most reactive towards nitratio...
Text Solution
|
- Which position will be attacked most rapidly by the nitronium ion (-NO...
Text Solution
|
- When is treated with nitrating mixture (HNO(3)+H(2)SO(4)), we get :
Text Solution
|
- Assertion(A):The nitrating reagent for carrying out nitration of benze...
Text Solution
|
- Phenol when nitrated with conc. HNO(3) in presence of conc. H(2)SO(4) ...
Text Solution
|
- Nitration of benzene is carried out with conc. HNO(3) in presence of c...
Text Solution
|