Similar Questions
Explore conceptually related problems
Recommended Questions
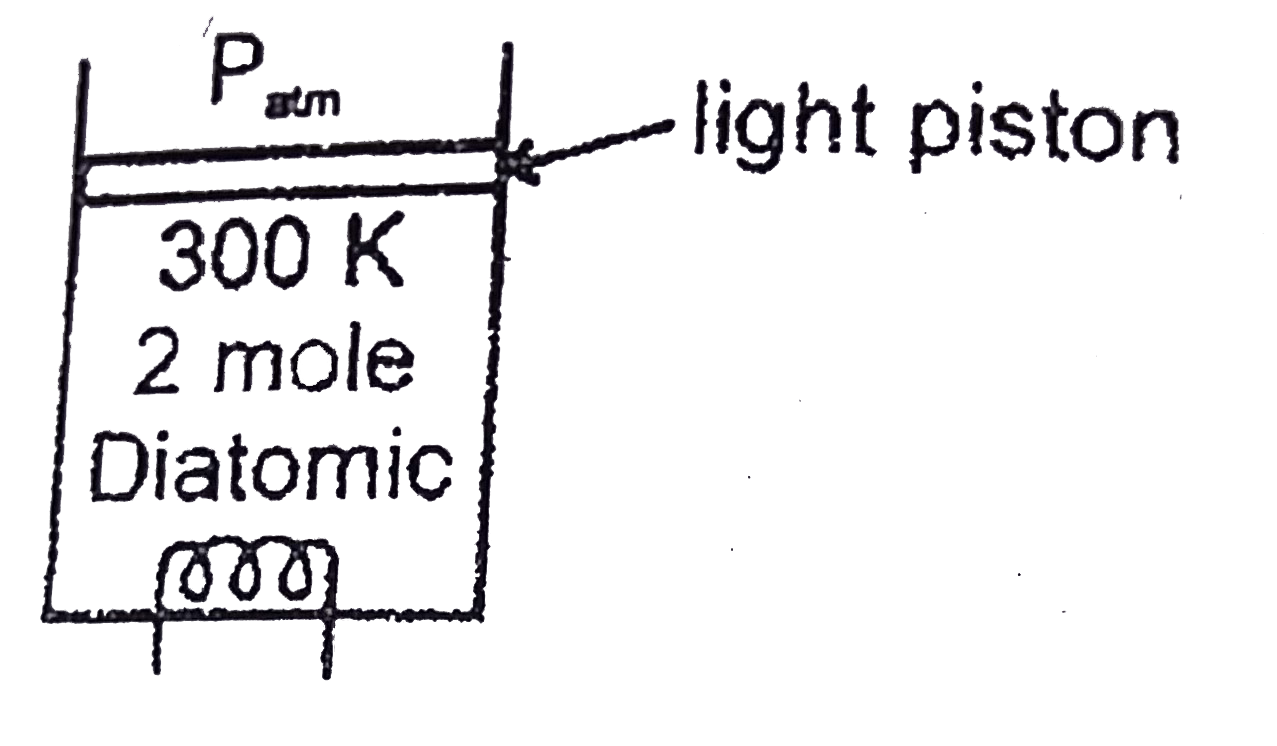
- Two moles of a diatomic gas at 300K are enclosed in a cylinder as show...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- A gas is filled in the cylinder shown in fig. The two pistons are join...
Text Solution
|
- Two moles of a diatomic gas at 300K are enclosed in a cylinder as show...
Text Solution
|
- In a cylindrical container two pistons enclose gas in two compartments...
Text Solution
|
- A horizontal cylinder has two sections of unequal cross - sections, in...
Text Solution
|
- 2 moles of a diatomic gas are enclosed in a cylinder piston arrangment...
Text Solution
|
- दो सिलिण्डरों A तथा B में जिनमें पिस्टन लगी है समान परिमाण को आदर्श द्...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|