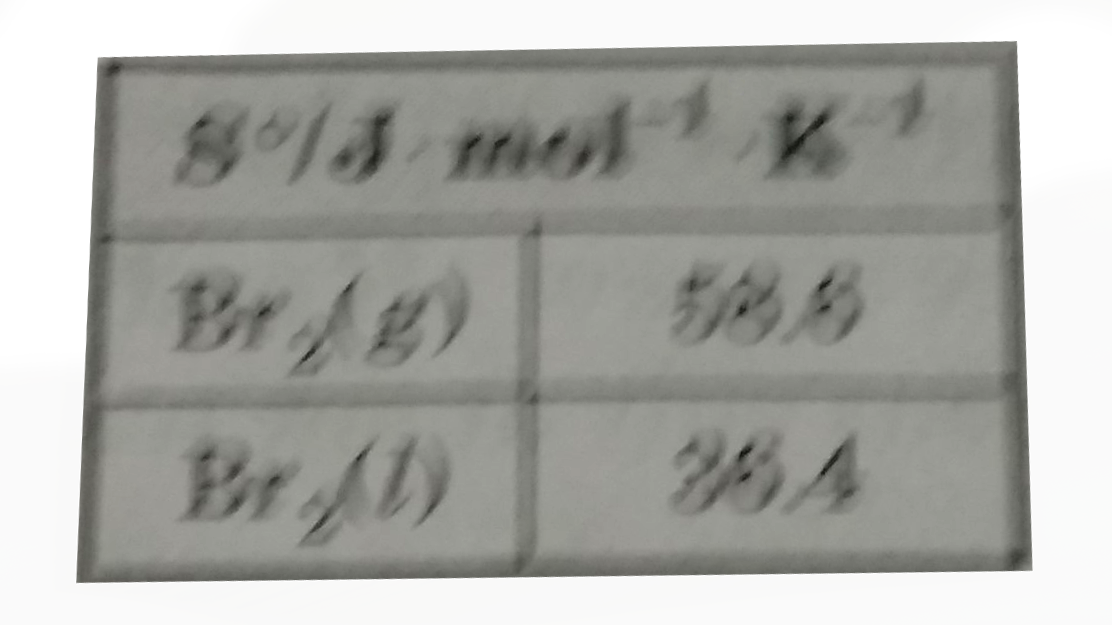Similar Questions
Explore conceptually related problems
Recommended Questions
- Liquid bromine boils at 332.7.K Estimate the enthalpy of formation of ...
Text Solution
|
- The enthalpy of vaporisation of a liquid is 30 kJ mol^(-1) and entropy...
Text Solution
|
- Liquid bromine boils at 332.7.K Estimate the enthalpy of formation of ...
Text Solution
|
- The standard enthalpy of formation of octane (C(8)H(18)) is -250kJ //m...
Text Solution
|
- The standard enthalpy of formation of H(2)(g) and Cl(2)(g) and HCl(g) ...
Text Solution
|
- The enthalpy of vaporisation of a liquid is 30 kJ mol^(-1) and entropy...
Text Solution
|
- मेथेन,ग्रेफाइट एवं डाईहाइड्रोजन के लिए 298 K पर दहन एन्थेलपी के मान क्...
Text Solution
|
- The enthalpy of combustion of methane, graphite and dihydrogen at 298 ...
Text Solution
|
- Enthalpy of combustion of benzene is -3267 kJ mol^(-1) . Calculate ent...
Text Solution
|