A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2010-Chemistry
- In chromite are , the oxidation number of iron and chromium respective...
Text Solution
|
- For the reversible reaction : A(s) + B(g) hArr C(g) + D(g) : Delta ...
Text Solution
|
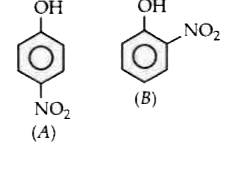
- Out of the two compounds below the vapour pressure of (B) at a particu...
Text Solution
|
- The amount of heat evolved when 500 cm^(3) of 0.1 M HCl is mixed with ...
Text Solution
|
- During the adsorption of krypton on activated charcoal at low temperat...
Text Solution
|
- The set of quantum numbers for the outermost electron for copper in it...
Text Solution
|
- Peroxide ion (i) has five completely filled antibonding molecular or...
Text Solution
|
- Which one of these is NOT true for benzene ?
Text Solution
|
- A mixture of CaCl(2) and NaCl weighing 4.44 g is treated with sodium c...
Text Solution
|
- For one mole of an ideal gas, increasing the temperature from 10^(@)C ...
Text Solution
|
- Generally, the first ionization enthalpy increases along a period. But...
Text Solution
|
- "50 cm"^(3) of 0.2" N HCl" is titrated against 0.1" N NaOH" The remai...
Text Solution
|
- In which one of the following, does the given amount of chlorine exert...
Text Solution
|
- Based on the first law of thermodynamics, which one of the following i...
Text Solution
|
- For alkali metals, which one of the following trends is incorrect ?
Text Solution
|
- One gram of silver gets distributed between "10 cm"^(3) of molten zinc...
Text Solution
|
- One mole of an organic compound A with the formula C(3)H(8)O reacts co...
Text Solution
|
- The IUPAC name of K(2)[Ni(CN)(4)] is :
Text Solution
|
- The spin only magnetic moment of Mn^(4+) ion is nearly
Text Solution
|
- In Kjeldahl's method, ammonia from 5g of food neutralizes 30cm^(3) of ...
Text Solution
|