A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-SOLVED PAPER 2011-CHEMISTRY
- Which one of these is NOT true for benzene ?
Text Solution
|
- Generally, the first ionization enthalpy increases along a period. But...
Text Solution
|
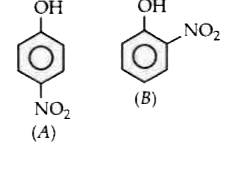
- Out of the two compounds below the vapour pressure of (B) at a particu...
Text Solution
|
- Increasing order of carbon-carbon bond length for the following is : ...
Text Solution
|
- A mixture of CaCl2 and NaCl weighing 4.44g is treated with sodium c...
Text Solution
|
- 50 cm^3 of 0.2N HCl is titrated against 0.1N NaOH solution. The titr...
Text Solution
|
- The r.m.s. velocity of hydrogen is sqrt( 7) times the r.m.s. velocity ...
Text Solution
|
- 25 g of each of the following gases are taken at 27^(@)C and 600 mm pr...
Text Solution
|
- The amount of heat evolved when 500 cm^(3) of 0.1 M HCl is mixed with ...
Text Solution
|
- Enthalpy of vaporization of benzene is + 35.3 kJ mol^(-1) at its boili...
Text Solution
|
- Based on the first law of thermodynamics, which one of the following i...
Text Solution
|
- Consider the following gaseous equilibria with equilibrium constants K...
Text Solution
|
- During the adsorption of krypton on activated charcoal at low temperat...
Text Solution
|
- For the reversible reaction : A(s) + B(g) hArr C(g) + D(g) : Delta ...
Text Solution
|
- Identify B and D in the following sequence of reactions
Text Solution
|
- The compound that reacts fastest with Lucas reagent is
Text Solution
|
- Ethyl benzene can not be prepared by :
Text Solution
|
- 1.2g of an organic compound on Kjeldahlization liberates ammonia which...
Text Solution
|
- Carbon can reduce ferric oxide to iron at a temperature above 983 K be...
Text Solution
|
- The yellow precipitate formed during the chromyl chloride test in chem...
Text Solution
|