A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-SOLVED PAPER 2011-CHEMISTRY
- Ethyl benzene can not be prepared by :
Text Solution
|
- 1.2g of an organic compound on Kjeldahlization liberates ammonia which...
Text Solution
|
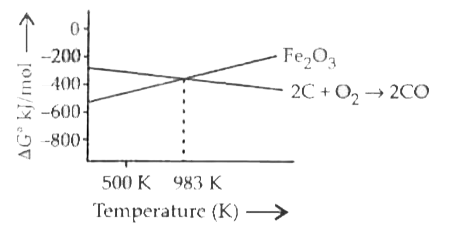
- Carbon can reduce ferric oxide to iron at a temperature above 983 K be...
Text Solution
|
- The yellow precipitate formed during the chromyl chloride test in chem...
Text Solution
|
- One gram of silver gets distributed between "10 cm"^(3) of molten zinc...
Text Solution
|
- Which one of the following is true?
Text Solution
|
- In Ramsay and Rayleigh's isolation of noble gases from air,the nitroge...
Text Solution
|
- The expected spin magnetic moment of Fe^(3+) is :
Text Solution
|
- Write the IUPAC name of the complex [Cr(NH3)4 Cl2] Cl.
Text Solution
|
- Excess of silver nitrate solution is added to 100 ml of 0.01 M penta a...
Text Solution
|
- The following data were obtained during the first order decomposition ...
Text Solution
|
- The time required for 100% completion of a zero order reaction is
Text Solution
|
- The activation energy for a reaction at the temperature T K was found...
Text Solution
|
- pH value of which one of the following is NOT equal to one ?
Text Solution
|
- A buffer solution contains 0.1 mole of sodium acetate dissolved in 100...
Text Solution
|
- H(2)S is passed into one dm^(3) of a solution containing 0.1 mole of Z...
Text Solution
|
- E(1),E(2) and E(3) are the emfs of the following three galvanic cells...
Text Solution
|
- 0.023g of sodium metal is reacted with 100 cm^(3) of water. The pH of ...
Text Solution
|
- The standard emf of a galvanic cell involving 2 moles of electrons in ...
Text Solution
|
- 9.65C of electric current is passed through fused anhydrous magnesium ...
Text Solution
|