Similar Questions
Explore conceptually related problems
Recommended Questions
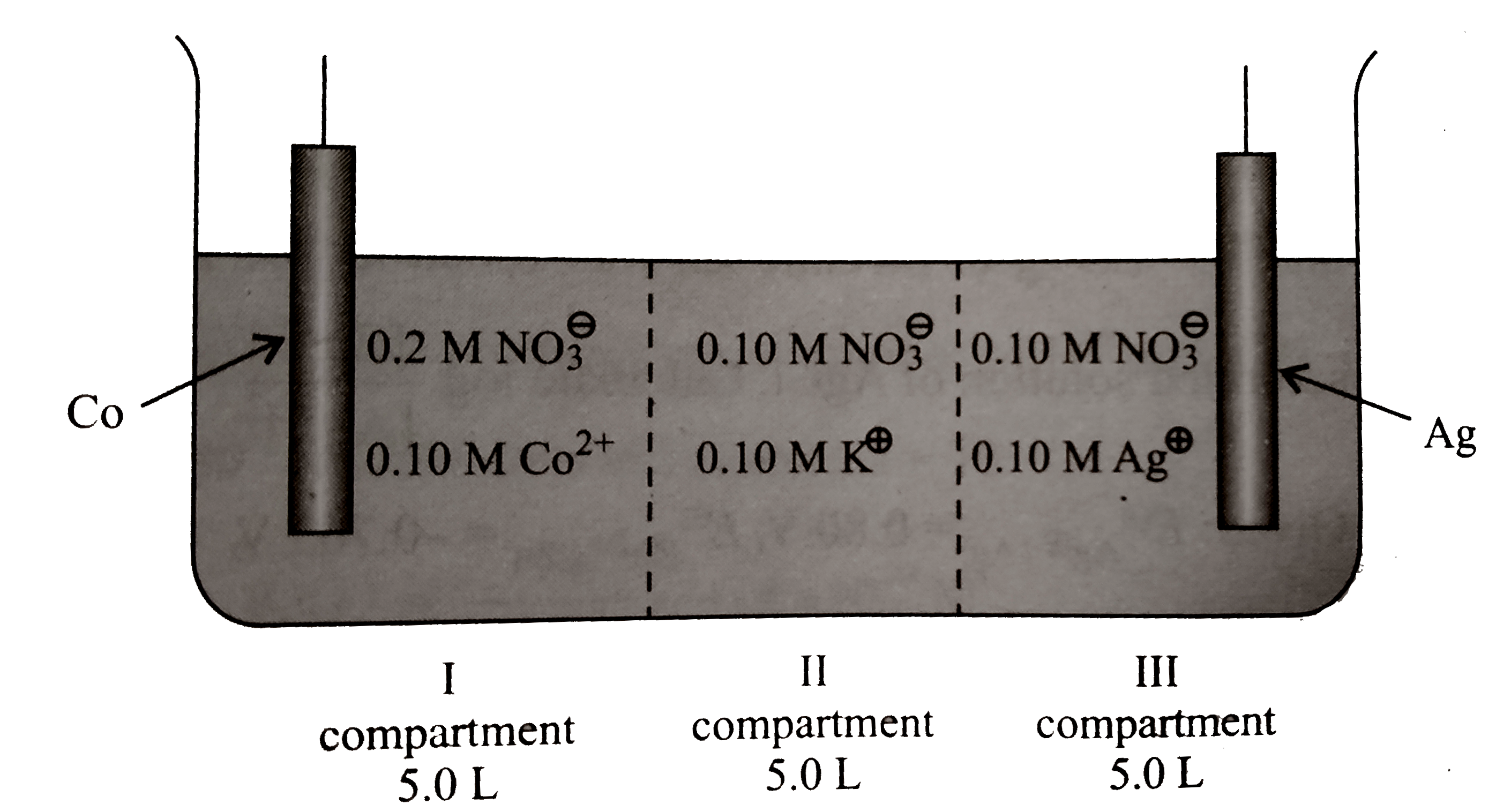
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|
- A volataic cell consists of an electode of solide silver immerse in a ...
Text Solution
|
- A volataic cell consists of an electode of solide silver immerse in a ...
Text Solution
|
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|
- A cell, as shown below, consists of there compartments separated by po...
Text Solution
|