Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion : is named as tetrakis ( ethylene diamine ) mu- hydroxo - i...
Text Solution
|
- In octaamine -mu -dihydroxodiiron(III)sulphate the number of bridging ...
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attched to cen...
Text Solution
|
- Ethylene diamine is an example of a ……… ligand:
Text Solution
|
- Assertion : is named as tetrakis ( ethylene diamine ) mu- hydroxo - i...
Text Solution
|
- Assertion : is named as tetrakis (ethylene- diammine) mu-"hydroxo"-mu-...
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attached to ce...
Text Solution
|
- Ethylene diamine is an example of a………. Ligand :
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attached to ce...
Text Solution
|
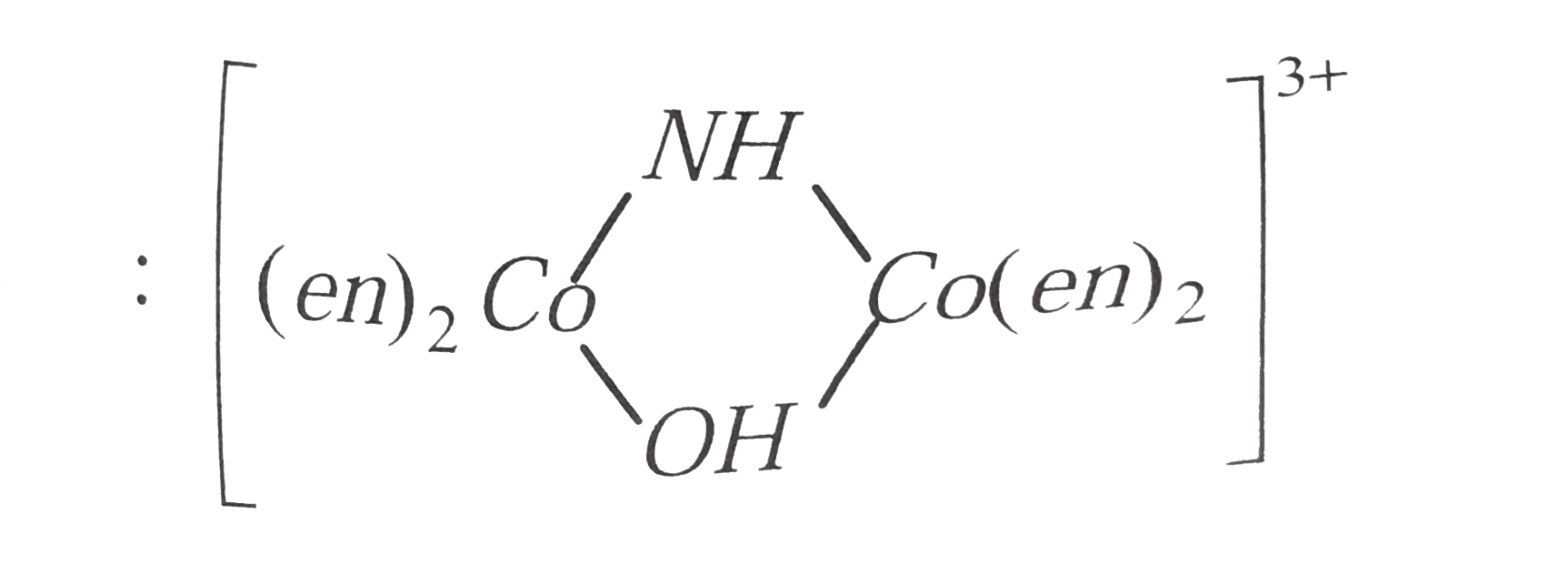 is named as tetrakis ( ethylene diamine ) `mu`- hydroxo - imido dicobalt (III) ion.
is named as tetrakis ( ethylene diamine ) `mu`- hydroxo - imido dicobalt (III) ion.