Similar Questions
Explore conceptually related problems
Recommended Questions
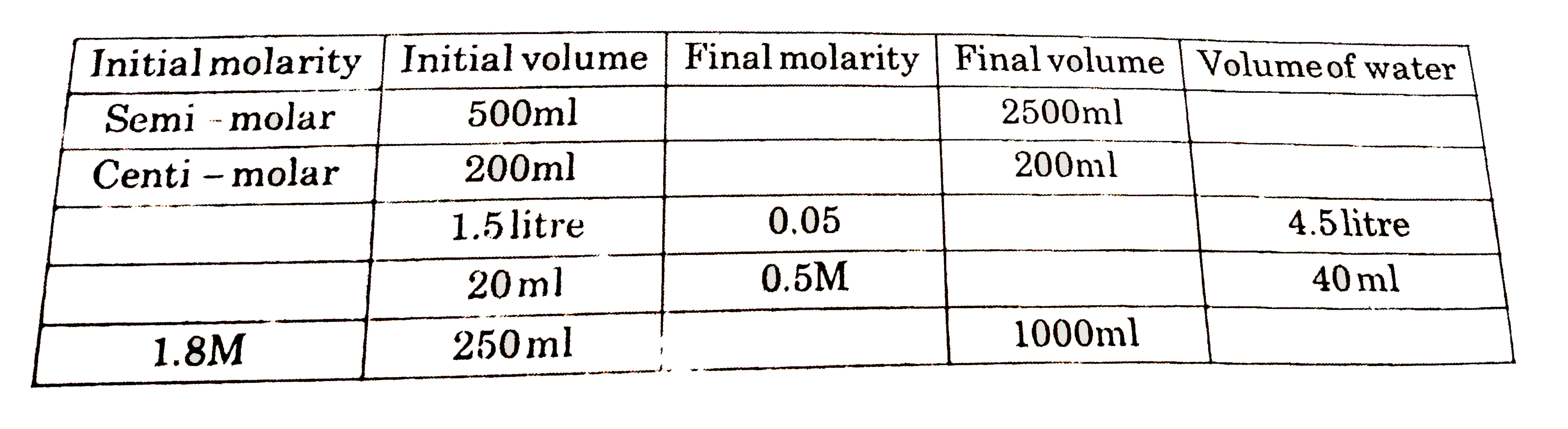
- Use of dilution formula (M(1)V(1) = M(2) V(2))
Text Solution
|
- Two masses, m(1) and m(2) , are moving with velocities v(1) and v(2). ...
Text Solution
|
- In a direct impact loss in kinetic energy is given by Delta K = (M(1...
Text Solution
|
- Two bodies P and Q of masses m(1) and m(2) (m(2) gt m(1)) are moving ...
Text Solution
|
- Two bodies having masses m(1) and m(2) and velocities v(1) and v(2) co...
Text Solution
|
- Use of dilution formula (M(1)V(1) = M(2) V(2))
Text Solution
|
- V(1) cc of solution having molarity M(1) is diluted to have molarity M...
Text Solution
|
- द्रव्यमान m(1) का एक पिण्ड अज्ञात वेग v(1)hati से चलते हुए एक-दूसरे द्...
Text Solution
|
- Figure 17.19 shows two different light-sensing substances. M(1) And M(...
Text Solution
|