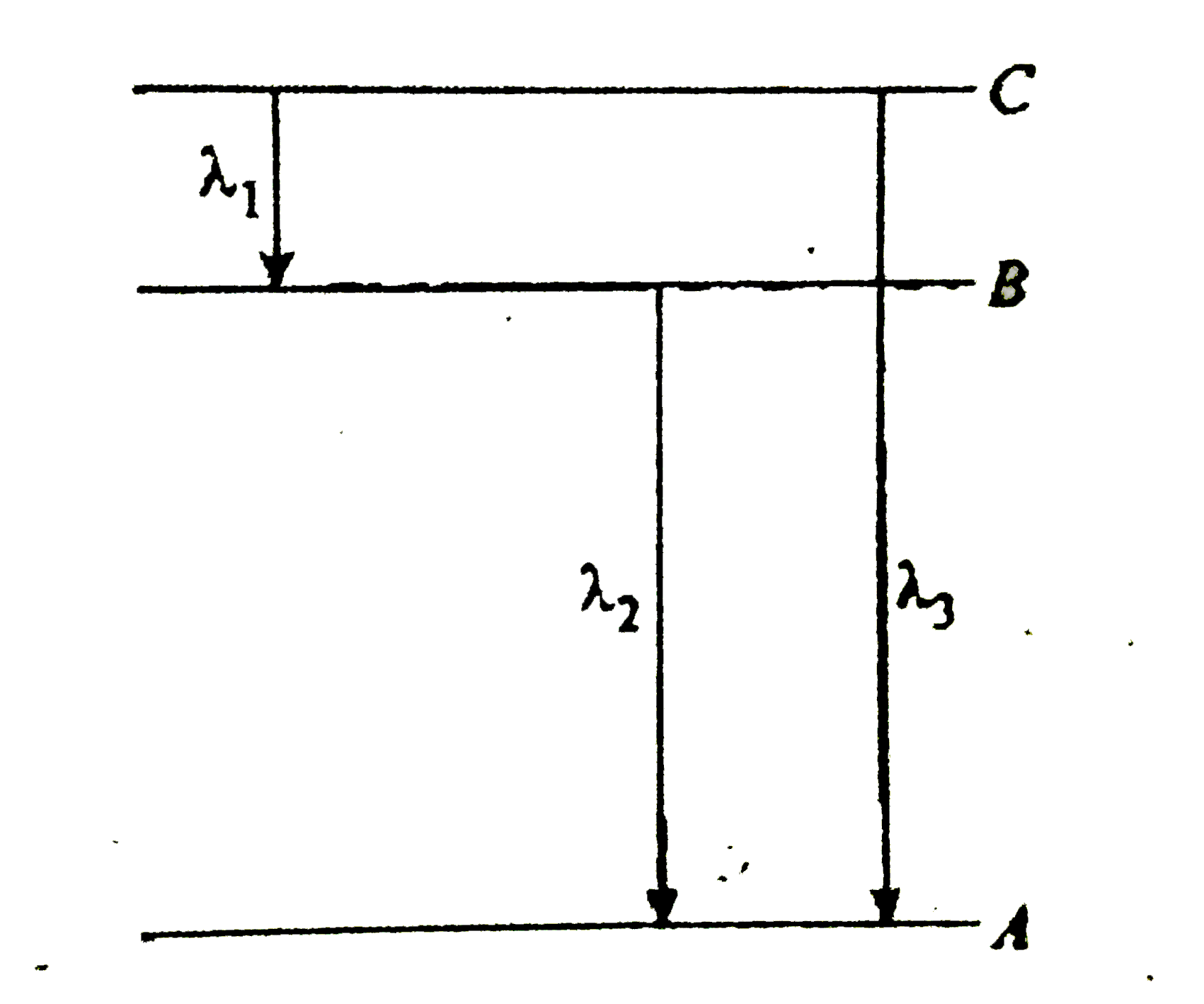
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-DUAL NATURE OF RADIATION AND MATTER-EXERCISE -2 : CONCEPT ALLPLICATOR
- A metal surface is illuminated by light of two different wavelengths 2...
Text Solution
|
- A beam of light has three wavelengths 4144 Å, 4972 Å and 6216 Å with a...
Text Solution
|
- Energy levels A,B and C of a certain atom correspond to increasing val...
Text Solution
|
- A copper ball of radius 1 cm and work function 4.47eV is irradiated wi...
Text Solution
|
- A parallel beam of electrons travelling in x-direction falls on a slit...
Text Solution
|
- Light of wavelength 0.6mum from a sodium lamp falls on a photocell and...
Text Solution
|
- An electron is accelerated through a potential difference of V volt. I...
Text Solution
|
- In a photoelectric emission, electrons are ejected from metals X and Y...
Text Solution
|
- A homogeneous ball (mass=m) of ideal black material at rest is illumin...
Text Solution
|
- Light of wavelength lamda from a small 0.5 mW He-Ne laser source, used...
Text Solution
|
- A photon and an electron posses same de-Broglie wavelength. Given that...
Text Solution
|
- Light of intensity I is incident perpendicularly on a perfectly reflec...
Text Solution
|
- What will be the ratio of de - Broglie wavelengths of proton and alpha...
Text Solution
|
- A sensor is exposed for time t to a lamp of power P placed at a distan...
Text Solution
|
- When X- ray of wavelength 0.5 Å pass through 7 mm thick aluminium shee...
Text Solution
|
- A source S(1) is producing 10^(15) photons/s of wavelength 5000 Å Anot...
Text Solution
|
- The potential difference that must be applied to stop the fastest phot...
Text Solution
|
- If the momentum of electron is changed by P(m) then the de-Broglie wa...
Text Solution
|
- Two radiations of photons energies 1 eV and 2.5 eV, successively illum...
Text Solution
|
- A modern 200 W sodium street lamp emits yellow light of wavelength 0.6...
Text Solution
|