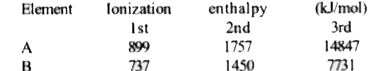A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DISHA PUBLICATION|Exercise Exercise -1 :Concept Builder (Topic 1)|21 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DISHA PUBLICATION|Exercise Exercise -1 :Concept Builder (Topic 2)|22 VideosCHEMISTRY IN EVERDAY LIFE
DISHA PUBLICATION|Exercise Exercise|88 VideosCOORDINATION COMPOUNDS
DISHA PUBLICATION|Exercise Exercise|122 Videos
Similar Questions
Explore conceptually related problems