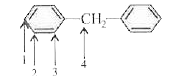
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 1 : Concept Builder (Topicwise) TOPIC 3 : Concepts of Reaction Mechanism in Organic Compounds and Purification|31 VideosORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 2 : Concept Applicator|30 VideosORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 1 : Concept Builder Builder (Topicwise) TOPIC 1 : Classification and Nomenclature of Organic Compounds)|22 VideosJEE MAIN 2019
DISHA PUBLICATION|Exercise MCQs|30 VideosPOLYMERS
DISHA PUBLICATION|Exercise EXERCISE -2 : CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES -Exercise - 1 : Concept Builder (Topicwise) TOPIC 2 : isomerism in Organic Compounds )
- The optically inactive compound from the following is :-
Text Solution
|
- Maleic acid and fumaric acid are :
Text Solution
|
- During debromination of meso- dibromobutane, the major compound forme...
Text Solution
|
- Which of the following is optically active
Text Solution
|
- Only two isomeric monochloro derivatives are possible for
Text Solution
|
- Which of the following is true ?
Text Solution
|
- The process of separation of racemic modifications into d and l enanti...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Geometrical isomers differ in:
Text Solution
|
- The number of chiral carbons in beta - D (+) - glucose is:
Text Solution
|
- which one of the following pairs represents the stereoisomerism?
Text Solution
|
- Which of the following will have a meso-isomer also ?
Text Solution
|
- An aromatic compound of formula C(7)H(7)Cl has in all ….. Isomers :
Text Solution
|
- How many optically active stereoisomers are possible for lactic acid ?
Text Solution
|
- Keto - enol tautomerism is oberved in :
Text Solution
|
- The molecular formula of diphenylmethane is How many structur...
Text Solution
|
- Which one of the following conformations of cyclohexane is chiral?
Text Solution
|