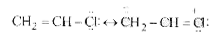A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 2 : Concept Applicator|30 VideosORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 1 : Concept Builder (Topicwise) TOPIC 2 : isomerism in Organic Compounds )|17 VideosJEE MAIN 2019
DISHA PUBLICATION|Exercise MCQs|30 VideosPOLYMERS
DISHA PUBLICATION|Exercise EXERCISE -2 : CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES -Exercise - 1 : Concept Builder (Topicwise) TOPIC 3 : Concepts of Reaction Mechanism in Organic Compounds and Purification
- Which one of the following orders is correct regarding the inductive e...
Text Solution
|
- Hyperconjugation is most useful for stabilizing which of the following...
Text Solution
|
- Chlorine in vinyl chloride is less reactive because
Text Solution
|
- In the following group : -OAc(I),-OMe(II),-OSO(2)(III),-OSO(2)CF(3)(...
Text Solution
|
- Most stable carbocation is :
Text Solution
|
- The decreasing order of nucleophilicity among the nucleophiles is : ...
Text Solution
|
- Decreasing order of reactivitytowards nucleophilic addition to carbon ...
Text Solution
|
- Select the appropriate relation with repect to acidity of X, Y, Z for ...
Text Solution
|
- the reaction is not possible because
Text Solution
|
- Which among the following group when attached to benzene ring will dir...
Text Solution
|
- In which of the following pairs A is more stable than B ?
Text Solution
|
- Which of the following has the highest nucleophilicity ?
Text Solution
|
- The increasing order of the boiling points for the following compound ...
Text Solution
|
- Which of the following compouns has most acidic hydrogen ?
Text Solution
|
- Polarization of electrons in acrolein may be written as:
Text Solution
|
- Identify most acidic hydrogen present in the above compound :
Text Solution
|
- Of the following compounds, which will have a zero dipole moment ?
Text Solution
|
- Which statement is incorrect in respect of the above reaction ?
Text Solution
|
- Carbon-carbon double bond lenghth will be maximum in which of the foll...
Text Solution
|
- The structure of CH(2)=C=CH(2) is :
Text Solution
|