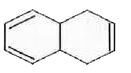
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 2 : Concept Applicator|30 VideosORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES
DISHA PUBLICATION|Exercise Exercise - 1 : Concept Builder (Topicwise) TOPIC 2 : isomerism in Organic Compounds )|17 VideosJEE MAIN 2019
DISHA PUBLICATION|Exercise MCQs|30 VideosPOLYMERS
DISHA PUBLICATION|Exercise EXERCISE -2 : CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUES -Exercise - 1 : Concept Builder (Topicwise) TOPIC 3 : Concepts of Reaction Mechanism in Organic Compounds and Purification
- In which of the following pairs A is more stable than B ?
Text Solution
|
- Which of the following has the highest nucleophilicity ?
Text Solution
|
- The increasing order of the boiling points for the following compound ...
Text Solution
|
- Which of the following compouns has most acidic hydrogen ?
Text Solution
|
- Polarization of electrons in acrolein may be written as:
Text Solution
|
- Identify most acidic hydrogen present in the above compound :
Text Solution
|
- Of the following compounds, which will have a zero dipole moment ?
Text Solution
|
- Which statement is incorrect in respect of the above reaction ?
Text Solution
|
- Carbon-carbon double bond lenghth will be maximum in which of the foll...
Text Solution
|
- The structure of CH(2)=C=CH(2) is :
Text Solution
|
- Which of the following will show aromatic behaviour ?
Text Solution
|
- Which of the following reactions cannot proceed by S(N)1 mechaism ?
Text Solution
|
- Ketene CH2=C=O has
Text Solution
|
- Which one of the following does not have sp^(2) hybridised carbon ?
Text Solution
|
- How many pi - bonds are present in naphthalene ?
Text Solution
|
- The shape of methyl carbanion is similar to that of -
Text Solution
|
- Which of the following represents the correct order of stability of ca...
Text Solution
|
- Which one of the following is most reactive towards electrophilic reag...
Text Solution
|
- The pair of electron in the given carbanion, CH(3)C -= C^(Θ), is prese...
Text Solution
|
- The major product of dehydraction of the following
Text Solution
|