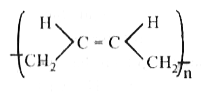
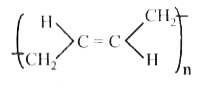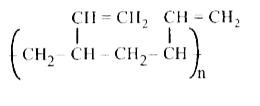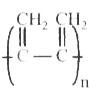A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-POLYMERS -EXERCISE -2 : CONCEPT APPLICATOR
- Synthetic polymer bakelite can be prepared from following compounds:-
Text Solution
|
- If a polythene sample contains two monodisperse fractions in the ratio...
Text Solution
|
- Mark out the most unlike form of polymerization of H(2)C=CH-CH=CH(2)
Text Solution
|
- The condensation of hexamethylenediamine with sebacoyl chloride at 525...
Text Solution
|
- Formation of polyethylene from calcium carbide takes place as follows ...
Text Solution
|
- Polymer formation from monomers starts by:
Text Solution
|
- Which of the following is fully fluorinated polymer?
Text Solution
|
- Among the following the wrong statement is
Text Solution
|
- In which of the following polymers , empirical formula resembles with ...
Text Solution
|
- Which is a polymer of three different monomers?
Text Solution
|
- The polymer which has conducting power is
Text Solution
|
- Orlon is a -
Text Solution
|
- Which of the following is not correct regarding terylene?
Text Solution
|
- Vinyl chloride can be converted into PVC.in this reaction the catalyst...
Text Solution
|
- The number average molecular mass and mass average molecular mass of a...
Text Solution
|
- The monomeric units of terylene are glycol and which of the following
Text Solution
|
- Given th polymers, A = Nylon-6,6, B= Buna-S, C = Polythene Arrange...
Text Solution
|
- Which one of the following statements is not true ?
Text Solution
|
- The compound which cannot be used as a plasticizer, is
Text Solution
|
- When condensation product of hexamethylenediamine and adipic acid is h...
Text Solution
|