Similar Questions
Explore conceptually related problems
Recommended Questions
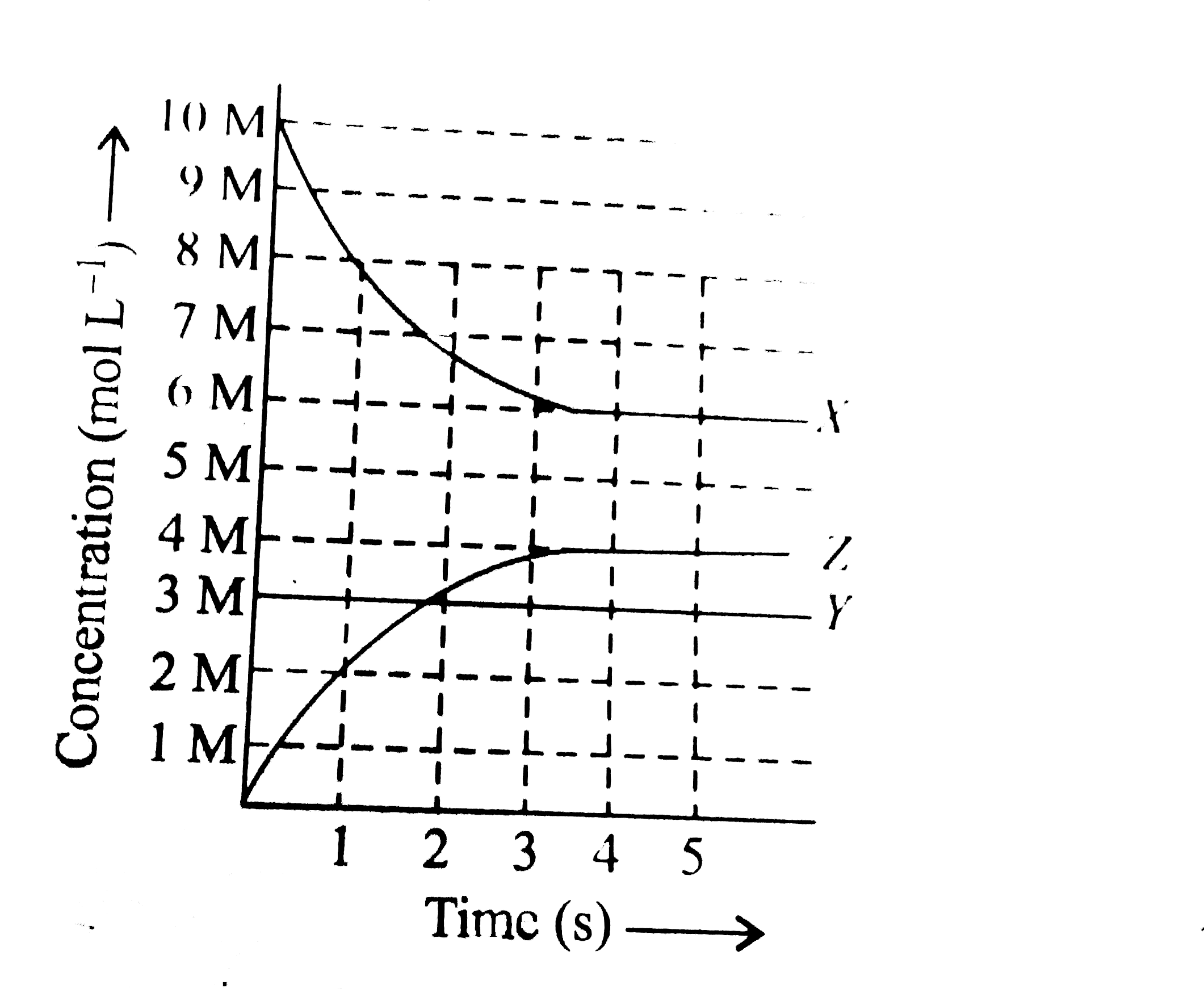
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentration ...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentration ...
Text Solution
|
- A,B and C react in the 1:1:1 stoichiometric ratio. The concentration o...
Text Solution
|
- A,B and C react in the 1:1:1 stoichiometric ratio. The concentration o...
Text Solution
|
- Silica reacts with magnesium compound X,X reacts with dil HCl and form...
Text Solution
|
- यदि tan^(-1)x,tan^(-1)y,tan^(-1)z समांतर श्रेढ़ी में हो तो x, y, z के ब...
Text Solution
|
- For the reaction A+2Brarr3C , the rate in terms of change in concentr...
Text Solution
|