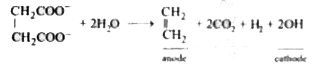A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-STATES OF MATTER-Exercise
- At very high pressure, the compressibility factor of one mole of a gas...
Text Solution
|
- Initially, the root mean square ( rms) velocity of N(2) molecules at c...
Text Solution
|
- Oxidation of succinate ion produces ethylene and carbon dioxide gases....
Text Solution
|
- Two closed bulbs of equal volume (V) containing an ideal gas initially...
Text Solution
|
- Which of the following is not an assumption of the kinetic theory of g...
Text Solution
|
- When does a gas deviate the most from its ideal behavior?
Text Solution
|
- The temperature at which oxygen molecules have the same root mean squa...
Text Solution
|
- van der Waal's equation for a gas is stated as, P=(nRT)/(V-nb)-a(n/V...
Text Solution
|
- The initial volume of a gas cylinder is 750.0 mL. If the pressure of g...
Text Solution
|
- The ratio of masses of oxygen and nitrogen in a particular gaseous mix...
Text Solution
|
- If Z is a compressibility factor, van der Waals' equation at low press...
Text Solution
|
- Dipole-dipole forces act between the molecules possessing permanent di...
Text Solution
|
- Gas equation PV = nRT is obeyed by
Text Solution
|
- Air at sea level is dense. This is a practical application of
Text Solution
|
- Equal volumes of gases at the same temperature and pressure contain eq...
Text Solution
|
- A gas in an open container is heated from 27^(@)C to 127^(@)C The frac...
Text Solution
|
- Initial temperature of an ideal gas is 75^(@)C. At what temperature, ...
Text Solution
|
- Two flasks A and B of 500 mL each are respectivelly filled with O(2)"...
Text Solution
|
- At what pressure a quantity of gas will occupy a volume of 60 mL, if i...
Text Solution
|
- The density of O(2)"(g)" is maximum at :
Text Solution
|