A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-THERMODYNAMICS-Exercise
- When 110 g of manganese (At mass =55) dissolves in dilute HNO(3) at 27...
Text Solution
|
- Work done for converson of 0.5 mole of water of 100^(@)C to steam at 1...
Text Solution
|
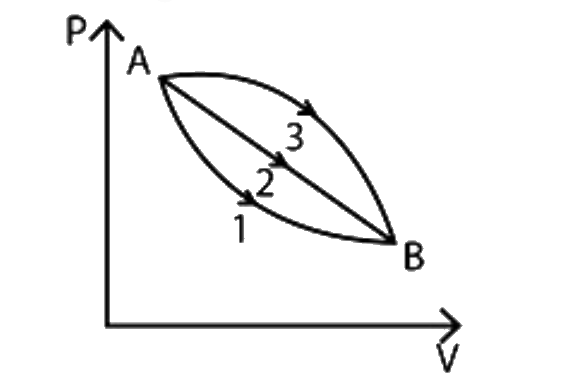
- A given mass of gas expands from state A to state B by three paths 1, ...
Text Solution
|
- If the door of a refrigerator is kept open, then which of the followin...
Text Solution
|
- 10 mole of ideal gas expand isothermally and reversibly from a pressur...
Text Solution
|
- One mole of an ideal gas (C(v,m)=(5)/(2)R) at 300 K and 5 atm is expan...
Text Solution
|
- Given that C+O(2)rarrCO(2),DeltaH^(@)=-xKJ and 2CO+O(2)rarr2CO(2),Delt...
Text Solution
|
- The heat of formations for CO(2)(g),H(2)O(l) and CH(4)(g) are -400kJ"m...
Text Solution
|
- The heat of neutralisation of strong base and strong acid is 57.0kJ/mo...
Text Solution
|
- If enthalpies of methane and enthane are respectively 320 and 560 calo...
Text Solution
|
- The heats of atomization of PH(3)(g) and P(2)H(4)(g) are 954 kJ "mol"^...
Text Solution
|
- Calculate the resonance enegry of N(2)O form the following data Delt...
Text Solution
|
- Enthalpy change for the reaction H^(+)(aq)+OH^(-)(aq)toH(2)O(l) is -...
Text Solution
|
- If enthalpy of combustion of carbon, hydrogen and C(3)H(8)" are "x(1),...
Text Solution
|
- The heat of sublimation of iodine is 24"cal"g^(-1) at 50^(@)C. If spec...
Text Solution
|
- The specific heat of a monoatomic gas at constant pressure is 248.2 J ...
Text Solution
|
- A steam boiler made up of steel weights 900 kg. The boiler contains 40...
Text Solution
|
- The enthalpy of neutraliztion of weak base A OH and a strong base BOH ...
Text Solution
|
- Two moles of ideal gas at 27^(@)C temperature is expanded reversibly f...
Text Solution
|
- Molar heat capacity of CD(2)O (deuterated form of formaldehyde) at con...
Text Solution
|