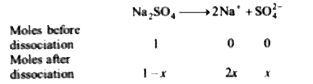A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-SOLUTIONS -Exercise
- Two 5 molal solution are prepared by dissoliving a non elecrtroyte no...
Text Solution
|
- For 1 molal aqueous solution of the following compounds, which one wil...
Text Solution
|
- 5 g of Na(2)SO(4) was dissolved in x g of H(2)O . The change in freezi...
Text Solution
|
- A solution is prepared by mixing 8.5g of CH(2)Cl(2) and 11.95g of CHCl...
Text Solution
|
- The freezing point of benzene decreases by 0.45^(@)C when 0.2 g of ace...
Text Solution
|
- The solubility of N(2) in water at 300K at 300K and 500 torr partial p...
Text Solution
|
- An aqueous solution of a salt MX(2) at certain temperature has a van'f...
Text Solution
|
- 18g glucose (C(6)H(12)O(6)) is added to 178.2g water. The vapour press...
Text Solution
|
- A solution at 20^(@)C is composed of 1.5 mol of benzene and 3.5 mol o...
Text Solution
|
- Determination of the molar mass of acetic acid in benzene using freezi...
Text Solution
|
- The vapor pressure of acetone at 20^(@)C is 185 torr. When 1.2 g of a ...
Text Solution
|
- Choose the correct statement with respect to the vapour pressure of a ...
Text Solution
|
- For an ideal solution of two components A and B, which of the followin...
Text Solution
|
- The observed osmotic pressure for a 0.10M solution of Fe(NH(4))(2)(SO(...
Text Solution
|
- Consider separate solution of 0.500M C(2)H(5)OH(aq), 0.100M Mg(3)(PO(4...
Text Solution
|
- When the solute is present in trace quantities the following expressio...
Text Solution
|
- Which one of the following gases has the lowest value of Henry law con...
Text Solution
|
- Equal moles of water and user are taken in a flask. What is mass perce...
Text Solution
|
- What is the normality of 1 M H(3)PO(4) solution ?
Text Solution
|
- Molarity of liquid HCl with density equal to 1.17g/cc is
Text Solution
|