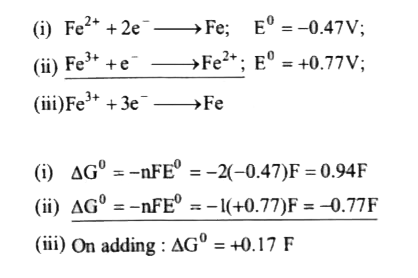A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ELECTROCHEMISTRY -Exercise
- When an electric current is passed through acidified water, 112ml of H...
Text Solution
|
- How long (approximate) should water be electrolysed by passing through...
Text Solution
|
- What is the standard reducing potential (E^(@)) for Fe^(3+)to Fe? (Gi...
Text Solution
|
- Consider the following standard electrode potentials (E^(@) in volts) ...
Text Solution
|
- Given E(Cl(2)//Cl^(-))^(@)=1.36V,E(Cr^(3+)//Cr)^(@)=-0.74V E("Cr(2)O...
Text Solution
|
- What will occur if a block of copper metal is dropped into a beaker co...
Text Solution
|
- Identify the correct statement
Text Solution
|
- Galvanization is applying a coating of:
Text Solution
|
- A variable, opposite external potential(E(ext) is applied to the ce...
Text Solution
|
- At 298 K, the standard reduction potentials are 1.51 V for MnO(4)^(-) ...
Text Solution
|
- Two faraday of electricity is passed through a solution of CuSO(4). Th...
Text Solution
|
- The standard electrode potentials (E(M^(+)//M)^(@)) of four metals A, ...
Text Solution
|
- A currrent of 5.0 A flows for 4.0 h through an electrlytic cell contai...
Text Solution
|
- Standard electrode potential data is given below : Fe^(3+) (aq) + e ...
Text Solution
|
- Resistance of 0.2 M solution of an electrolyte is 50Omega . The specif...
Text Solution
|
- The equivalent conductance of NaCl at concentration of C and at infini...
Text Solution
|
- Given below are the half -cell reactions Mn^(2+)+2e^(-) to Mn, E^(@)...
Text Solution
|
- The function of a salt bridge is to:
Text Solution
|
- Standard electrode potential of SHE at 298 K is :
Text Solution
|
- The satandard EMF of quinhydrone is 0.699V. The EMF of the quinhydrone...
Text Solution
|