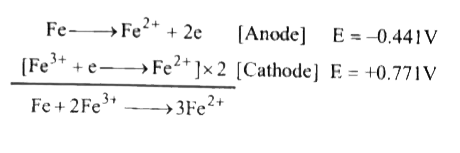A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ELECTROCHEMISTRY -Exercise
- Standard electrode potential of SHE at 298 K is :
Text Solution
|
- The satandard EMF of quinhydrone is 0.699V. The EMF of the quinhydrone...
Text Solution
|
- If e(Fe^(2+) //Fe)^@ =- 0. 44 1V. And E(Fe^(3+)//Fe^(2+))^(o) = 0. 771...
Text Solution
|
- In the electrolytic cell, flow of electrons is form :
Text Solution
|
- Given E(Cr^(3+)//cr)^@ =- 0.72 V, E(Fe^(2+)//Fe)^@ =- 0.42 V. The pote...
Text Solution
|
- Which of the following reaction is possible at anode ?
Text Solution
|
- Which one is not called a anode reaction from the following?
Text Solution
|
- Which of the following statements is true for an electrochemical cell ...
Text Solution
|
- At 298 K the standard free energy of formation of H(2)O(l) is -237.20 ...
Text Solution
|
- If the following half cells have E^(@) values as A^(3+) + e^(-) to A...
Text Solution
|
- In a galvanic cell
Text Solution
|
- Based on the cell notation for a spontaneous reaction, at the anode: ...
Text Solution
|
- Zn can displace :-
Text Solution
|
- The oxidation potential of a hydrogen electrode at pH = 10 and P(H(2))...
Text Solution
|
- The E^(@) at 25^(@)C for the following reaction is 0.22 V. Calculate t...
Text Solution
|
- A galvanic cell is composed of two hydrogen electrods, one of which i...
Text Solution
|
- The solution of CuSO(4) in which copper rod is immersed is diluted to ...
Text Solution
|
- For a given reaction :M^((x+n))+n e^(-)rarr M^(c+),E^(c-).(red) is kn...
Text Solution
|
- In the electrochemical reaction, 2Fe^(3+)+Zn rarr Zn^(2+) + 2Fe^(2+)...
Text Solution
|
- What is the standard reducing potential (E^(@)) for Fe^(3+)to Fe? (Gi...
Text Solution
|