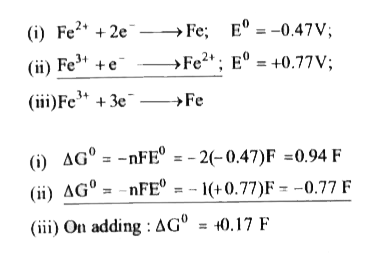A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ELECTROCHEMISTRY -Exercise
- For a given reaction :M^((x+n))+n e^(-)rarr M^(c+),E^(c-).(red) is kn...
Text Solution
|
- In the electrochemical reaction, 2Fe^(3+)+Zn rarr Zn^(2+) + 2Fe^(2+)...
Text Solution
|
- What is the standard reducing potential (E^(@)) for Fe^(3+)to Fe? (Gi...
Text Solution
|
- What will be the emf for the given cell ? Pt|H(2)(g,P(1))|H^(+)(aq)|...
Text Solution
|
- Ni|Ni^(2+)(1.0M)||Au^(3+)(1.0M)| Au (where E^(@) for Ni^(2+)//Niis -0...
Text Solution
|
- For a cell reaction involvinig a two electron change, the standard emf...
Text Solution
|
- Following cell has EMF 0.7995 V . Pt| H(2) (1) atm) |HNO(3) (1M)|| A...
Text Solution
|
- An electrochemical cell is shown below Pt, H(2)(1 "atm")|HCl(0.1 M)|C...
Text Solution
|
- The emf of the cell Cr(s) abs(Cr^(3+)(1.0M))abs(Co^(2+)(1.0M))Co(s) [E...
Text Solution
|
- Define the following. (a) stable equilibrium (b) unstable equilibr...
Text Solution
|
- Equivalent conductivity can be expressed in terms of specific conducta...
Text Solution
|
- The molar conducatance of Ba^(2+) and Cl^(-) are 127 and 76 ohm^(-1) c...
Text Solution
|
- The increases in equivalent conductivity of a weak electrolyte with di...
Text Solution
|
- HNO3(aq) is titrated with NaOH(aq) condutomatrically, graphical repres...
Text Solution
|
- If X is the specific resistance of the solution and N is the normality...
Text Solution
|
- For an electrolyte solution of 0.05 mol L^(-1) , the conductivity has...
Text Solution
|
- What is the value of pK(b)(CH(3)COO^(c-)) if wedge^(@).(m)=390 S cm^(-...
Text Solution
|
- An increase in equivalent conductance of a strong electrolyte with dil...
Text Solution
|
- The correct order of equivalent conductance at infinite dilution of Li...
Text Solution
|
- If 0.01 M solution of an electrolyte has a resistance of 40 ohms in a ...
Text Solution
|