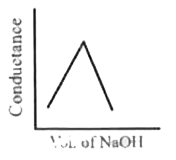
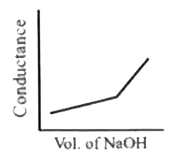
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ELECTROCHEMISTRY -Exercise
- The molar conducatance of Ba^(2+) and Cl^(-) are 127 and 76 ohm^(-1) c...
Text Solution
|
- The increases in equivalent conductivity of a weak electrolyte with di...
Text Solution
|
- HNO3(aq) is titrated with NaOH(aq) condutomatrically, graphical repres...
Text Solution
|
- If X is the specific resistance of the solution and N is the normality...
Text Solution
|
- For an electrolyte solution of 0.05 mol L^(-1) , the conductivity has...
Text Solution
|
- What is the value of pK(b)(CH(3)COO^(c-)) if wedge^(@).(m)=390 S cm^(-...
Text Solution
|
- An increase in equivalent conductance of a strong electrolyte with dil...
Text Solution
|
- The correct order of equivalent conductance at infinite dilution of Li...
Text Solution
|
- If 0.01 M solution of an electrolyte has a resistance of 40 ohms in a ...
Text Solution
|
- The resistance of 0.01 N solution of an electrolyte was found to be 22...
Text Solution
|
- 0.05 M NaOH solution offered a resistance of 3.1*6Omega in a conductiv...
Text Solution
|
- The specific conductance of a 0.1 N KCl solution at 23^(@)C is 0.012 o...
Text Solution
|
- Which of the following is an electrolyte ?
Text Solution
|
- The electric charge required for electrode deposition of one gram-equi...
Text Solution
|
- How many minutes will it take to plate out 5.2g of cr from a Cr2(SO4)3...
Text Solution
|
- Calculate the current (in mA) required to deposite 0.195g of platinum ...
Text Solution
|
- By virtue of Faraday's second law of electrolysis, the electrochemical...
Text Solution
|
- In electrolysis of very dilute of NaOH using platinum electrodes
Text Solution
|
- Indicate the reactions which take place at cathode and anode in fuel c...
Text Solution
|
- A hydrogen gas electrode is made by dipping platinum wire in a solutio...
Text Solution
|