A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-ELECTROCHEMISTRY -Exercise
- Consider the following equation for an electrochemical cell reaction. ...
Text Solution
|
- The temperature coefficient of a cell whose operation is based on the ...
Text Solution
|
- A fuel cell develops an electrical potential from the combustion of bu...
Text Solution
|
- Given the following cell at 25^(@) C . What will be the potential o...
Text Solution
|
- The combustion of butane in O(2) at 1 bar and 298K shows a decrease in...
Text Solution
|
- The emf of the cell, Zn|Zn^(2+) (0.01 M)||Fe^(2+) (0.001 M)|Fe at 29...
Text Solution
|
- The specific conductance of a saturated solution of silver bromide is ...
Text Solution
|
- Given the ionic conductance of underset(underset(COO^(c-))(|))(COO^(c-...
Text Solution
|
- During conductometric titration of 0. 1 MHCl with 1.0 M KOH, which is ...
Text Solution
|
- What is the amount of chlorine evoled when 2 amperes of current is pas...
Text Solution
|
- The specific conductance of a 0.1 N KCl solution at 23^(@)C is 0.012 o...
Text Solution
|
- lambda(ClCH(2)COONa)=224 "ohm"^(-1) cm^(2) " gm eq"^(-1), lambda(Na...
Text Solution
|
- Calculate Lambda(HO Ac)^(oo) using appropriate molar conductances of t...
Text Solution
|
- An aqueous solution containing 1M each of Au^(3+),Cu^(2+),Ag^(+),Li^(...
Text Solution
|
- A 100.0mL dilute solution of Ag^(+) is electrolysed for 15.0 minutes w...
Text Solution
|
- A 1 M solution of H2SO4 is electrolysed. Select correct statement in r...
Text Solution
|
- The value of standard hydrogen electrode potential is taken as zero, b...
Text Solution
|
- Faraday's Law of Electrolysis
Text Solution
|
- Sodium is made by the electrolysis of a molten mixture of about 40% Na...
Text Solution
|
- A lead storage battery containing 5.0 L of 1N H(2)SO(4) solution is op...
Text Solution
|
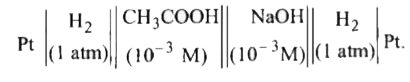 . What will be the potential of the cell ? Given `pK_(a)` of `CH_(3) COOH = 4.74`
. What will be the potential of the cell ? Given `pK_(a)` of `CH_(3) COOH = 4.74`