A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-THE P-BLOCK ELEMENTS (GROUP 15, 16, 17 & 18)-Exercise
- [X] +H(2)SO(4) to [Y] colourless with irritating smell. [Y] +K(2)Cr(2...
Text Solution
|
- The correct order of O - O bond length in O(2)H(2)O(2) and O(3) is
Text Solution
|
- Identify the correct sequence of increasing number of pi-bonds in the ...
Text Solution
|
- The correct order of S-S bod length is following oxyanions is : (i) ...
Text Solution
|
- Which of the following statements is false?
Text Solution
|
- Oxygen and sulphur both are the members of the same group in periodic ...
Text Solution
|
- A greenish yellow gas reacts with an alkali metal hydroxide to form a ...
Text Solution
|
- Which one of the following pairs of reactantas does not form oxygen wh...
Text Solution
|
- Which of the following is correct statement?
Text Solution
|
- Only iodine forms hepta-fluroide IF(7), but chlorine and bromine give ...
Text Solution
|
- The incorrect order is
Text Solution
|
- Which of the following oxyacids of chlorine is formed on shaking chlor...
Text Solution
|
- The correct statement(s) regarding, (i) HClO, (ii) HClO(2), (iii) HC...
Text Solution
|
- The formation of O(2)^(+)[PtF(6)]^(-) is the basis for the formation o...
Text Solution
|
- Inert gases such as heliume behave like ideal gases over a wide rang...
Text Solution
|
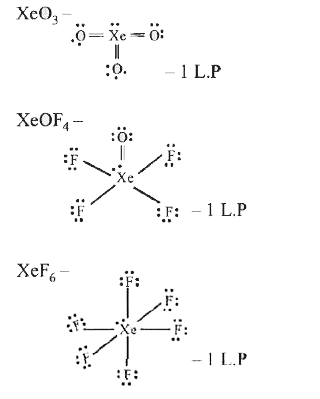
- Out of (i) XeO(3)(ii) XeOF(4) and (iii) XeF(6) the molecules having sa...
Text Solution
|
- Noble gases can be separated by:
Text Solution
|
- XeF(2) and XeF(6) are separately hydrolysed then:
Text Solution
|
- SbF5 react with XeF4 to form an adduct. The shapes of cation and anion...
Text Solution
|
- Which one of the following statements regarding helium is incorrect?
Text Solution
|