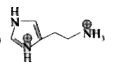
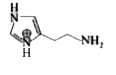
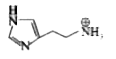
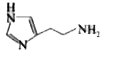A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-CHEMISTRY IN EVERDAY LIFE -Exercise
- The correct match between items of List - I and List - II is :
Text Solution
|
- The predominant form of histamine present in human blood is (rho K...
Text Solution
|
- Which of the following is a bactericidal antibiotic?
Text Solution
|
- Which of the following is an anionic detergent?
Text Solution
|
- Which artificial sweetener contains chlorine?
Text Solution
|
- Which of the following compounds is not an antacid?
Text Solution
|
- Which one of the following is used as Antihistamine?
Text Solution
|
- Aminoglycosides are usually used as :
Text Solution
|
- An anitpyretic is
Text Solution
|
- Salol can be used as
Text Solution
|
- Which one of the following is employed as a tranquilizer ?
Text Solution
|
- Terfenadine is commonly used as a/an
Text Solution
|
- Tranquillizers are substances used for the treatment of :
Text Solution
|
- Which of the following is used for inducing sleep?
Text Solution
|
- Chloramine - T is a/an.
Text Solution
|
- Penicilin was first discovered by .
Text Solution
|
- Which of the following terms means pain killer ?
Text Solution
|
- Which of the following hormones is produced under the conditions of st...
Text Solution
|
- Arsenic containing medicine used for the treatment of syphilis is...
Text Solution
|
- Which of the following is an insecticide?
Text Solution
|