Similar Questions
Explore conceptually related problems
Recommended Questions
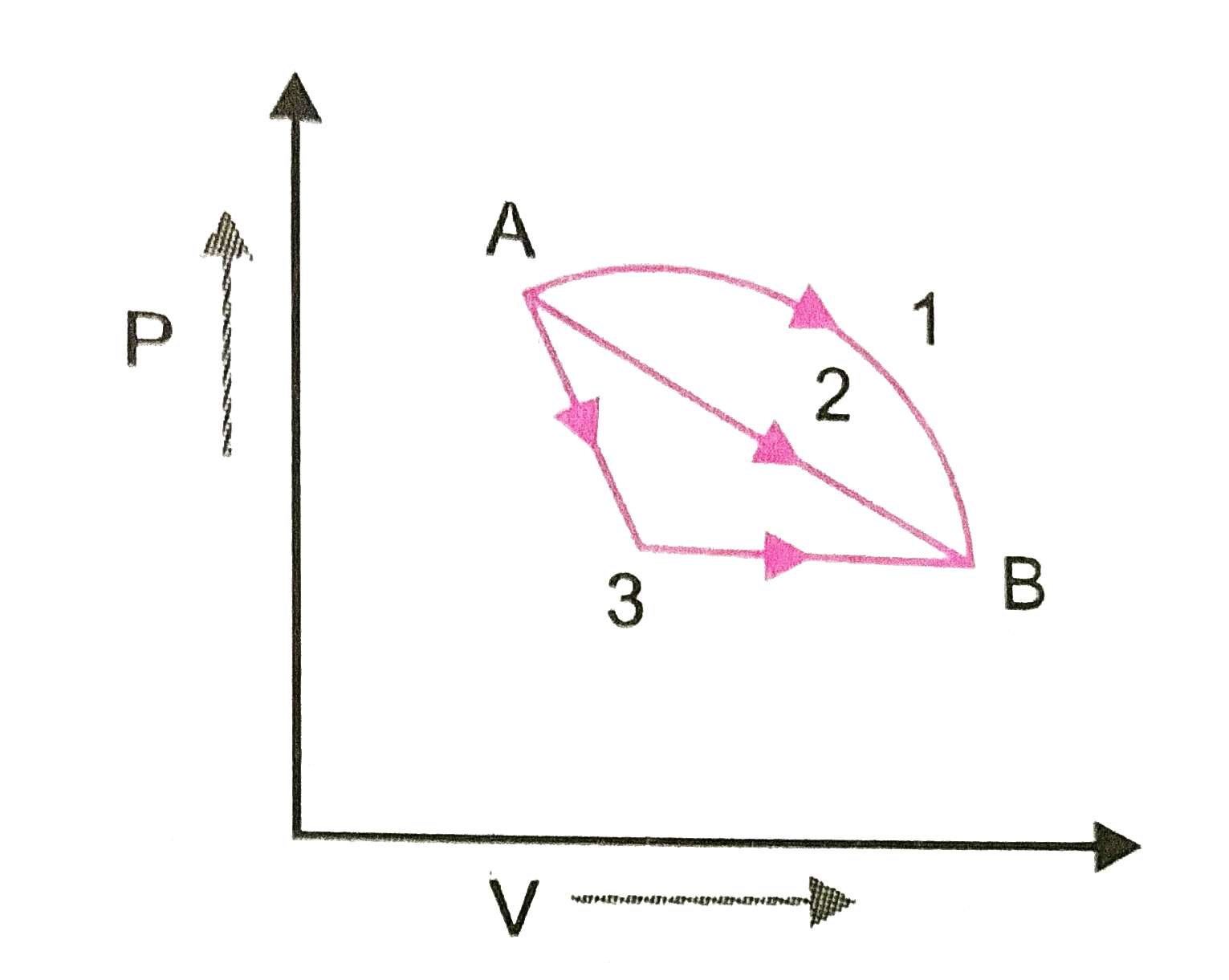
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- A gas is expanded form volume V(0) to 2V(0) under three different proc...
Text Solution
|
- दिखाए गए P-V आरेख के अनुसार एक आदर्श गैस को तीन विभिन्न प्रक्रमों द्...
Text Solution
|
- The initial state of 1 mol of an ideal gas is (P(1),V(1),T(1)) . The g...
Text Solution
|
- A gas is expanded from volume V(0) to 2V(0) under three different proc...
Text Solution
|
- An ideal gas goes from state A to state B via three different processe...
Text Solution
|
- चित्र में गैस के सेम्पल के लिए दो प्रक्रम a व b दिये गये हैं। यदि Delt...
Text Solution
|