Similar Questions
Explore conceptually related problems
Recommended Questions
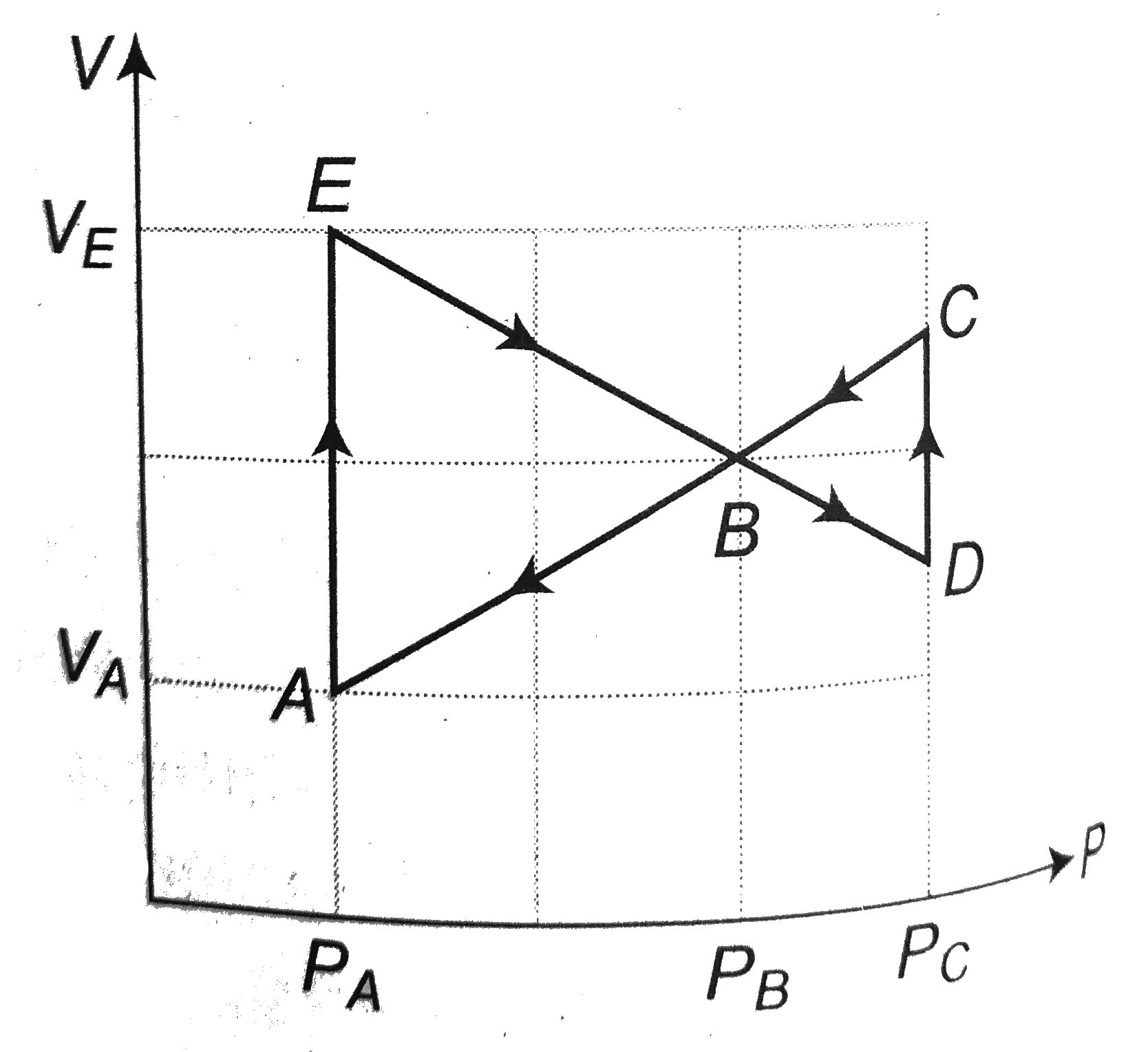
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If ...
Text Solution
|
- Determine the work done by an ideal gas doing 1 rarr 4 rarr 3rarr 2 ra...
Text Solution
|
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If ...
Text Solution
|
- The initial volume of an ideal gas is V(1) and the initial pressure is...
Text Solution
|
- An ideal monoatomic gas is initially in state 1 with pressure p(1) = 2...
Text Solution
|
- An ideal monoatomic gas initially in state 1 with pressure P(1)=20 atm...
Text Solution
|
- An ideal gas was subjected to following process: (n(1)=2"mole",T(1)=40...
Text Solution
|
- If a gas at a pressure of 10 atm at 300 K expands against a constant e...
Text Solution
|
- In the figure shown below, an ideal gas is carried around the cyclic p...
Text Solution
|