Similar Questions
Explore conceptually related problems
Recommended Questions
- Statement-1: is more stable Statement-2: Torsional strain and flag ...
Text Solution
|
- a. Draw (i) half chair, (ii) skew or twist boat cyclohexane conformati...
Text Solution
|
- How many of the following statements are correct? 1) The most stable c...
Text Solution
|
- Stetement-1: Boat fromis the least stable conformation of cyclohexane....
Text Solution
|
- Statement-1: is more stable Statement-2: Torsional strain and flag pol...
Text Solution
|
- Which of the following conformers of n-butane has torsional strain?
Text Solution
|
- The energy difference between the chair and the boat conformations of ...
Text Solution
|
- For cyclohexane, which of the following factors does not make the boat...
Text Solution
|
- Statement-1. The guache conformation of ethylene glycol is more stable...
Text Solution
|
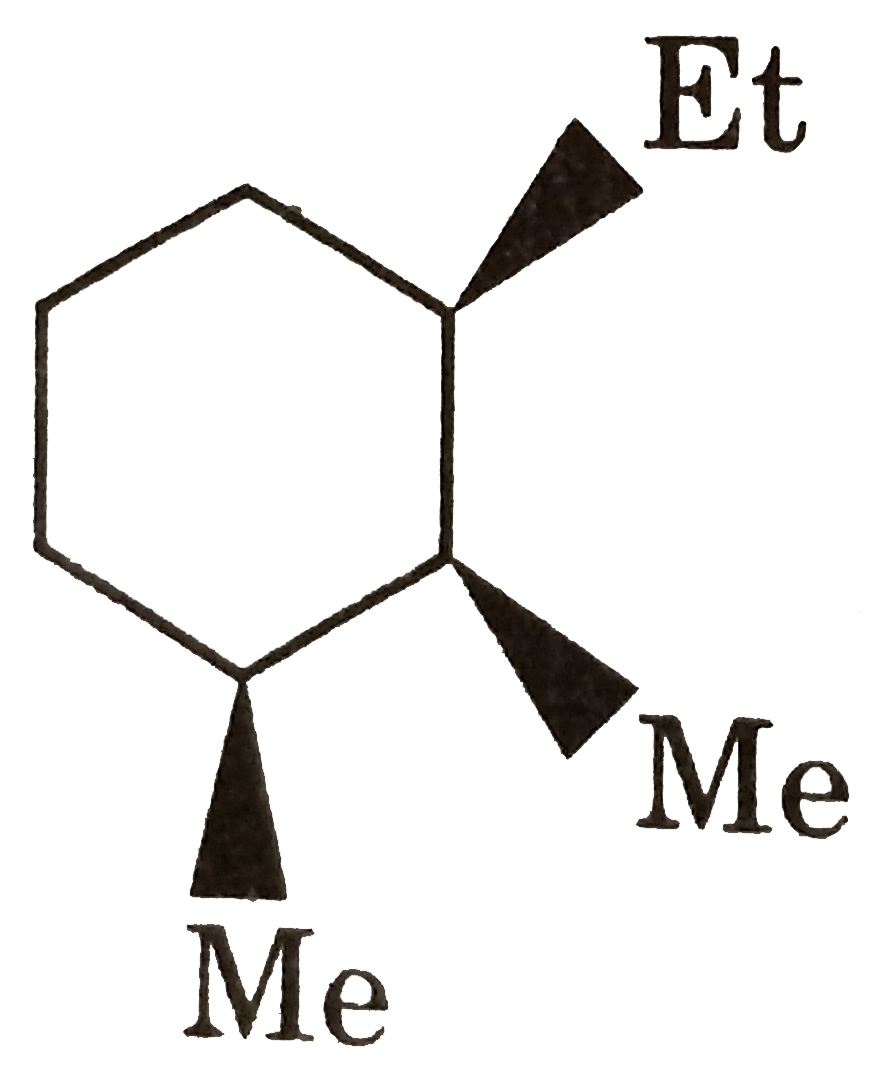 is more stable
is more stable