Similar Questions
Explore conceptually related problems
Recommended Questions
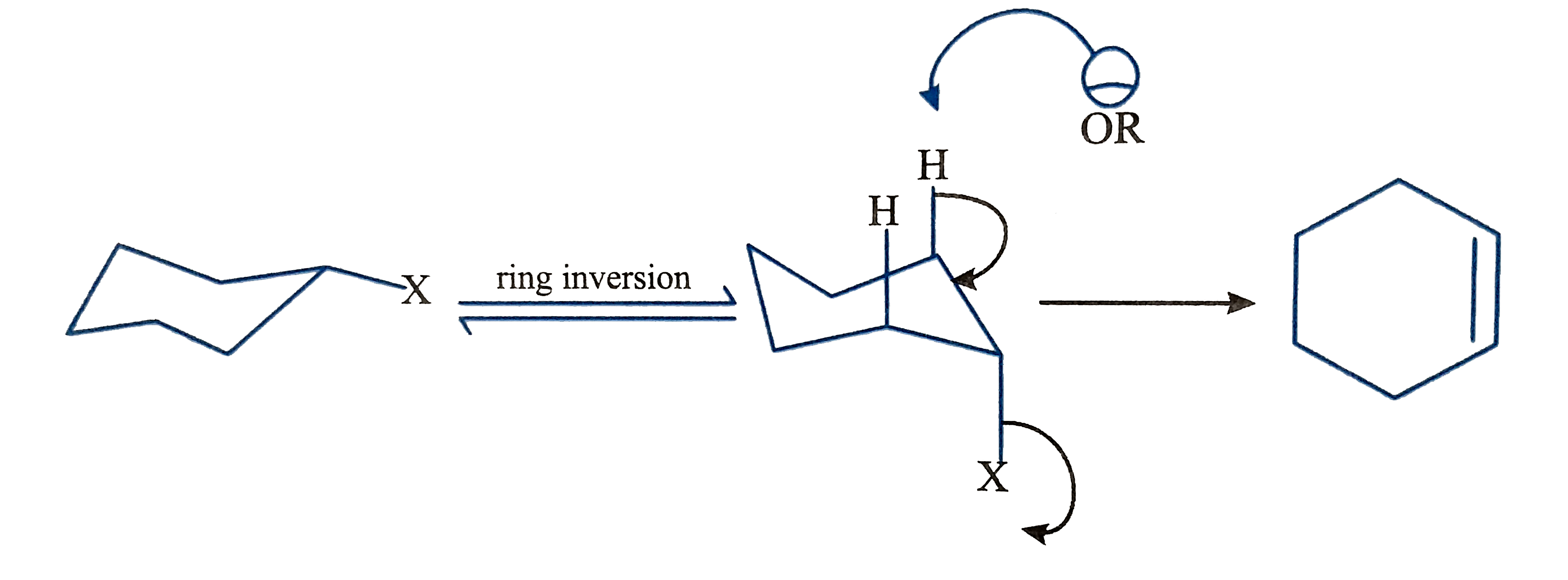
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- The the most stable conformer of cis-cyclohexan-1,4-diol is:
Text Solution
|
- The most stable conformer cis-cyclohexan-1,4-diol is:
Text Solution
|
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- The most stable conformation of cyclohexane is :
Text Solution
|
- Cyclohexane exist as two chair conformations in rapid equilibrium at r...
Text Solution
|
- Stability of cycloalkanes can be explained on the basis of Bayer stain...
Text Solution
|