Similar Questions
Explore conceptually related problems
Recommended Questions
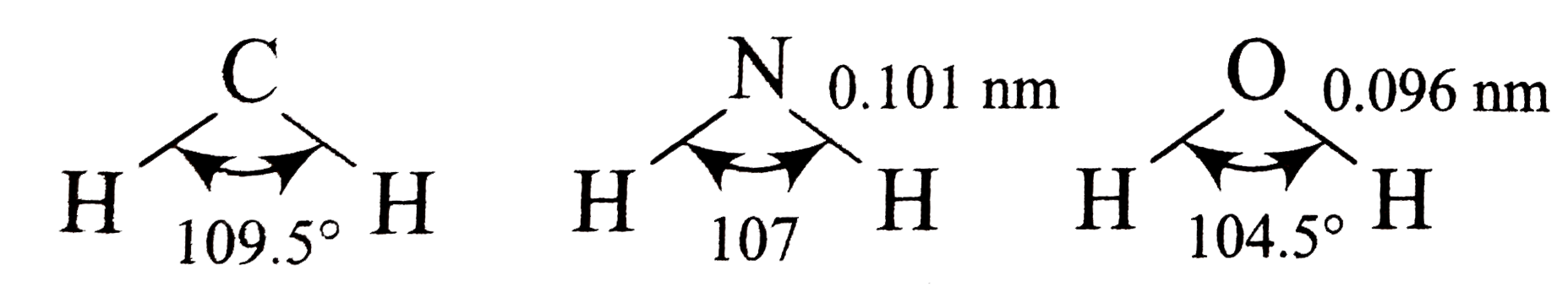
- The bond lengths and bond angles in the molecules of methane, ammonia,...
Text Solution
|
- The bond lengths and bond angles in the molecules of methane, ammonia,...
Text Solution
|
- According VSEPR theory, in the electronic geometry of the molecule(s),...
Text Solution
|
- According VSEPR theory, in the electronic geometry of the molecule(s),...
Text Solution
|
- Why is the repulsion between two lone pairs of electrons more than tha...
Text Solution
|
- कथन : NH(3) में प्रेक्षित आबन्ध कोण 109^(@)28' से कम होता है । ...
Text Solution
|
- In a bonded molecule, the order of repulsion between the bonded and no...
Text Solution
|
- The bond lengths and bond angles in the molecules of methane , ammo...
Text Solution
|
- The space model which is obtained by joining the points representing v...
Text Solution
|