Similar Questions
Explore conceptually related problems
Recommended Questions
- 1.0 k-mol of a sample of helium gas is put through the cycle of operat...
Text Solution
|
- The value of (Cp - Cv) is 1.00 R for a gas sample in state A and is ...
Text Solution
|
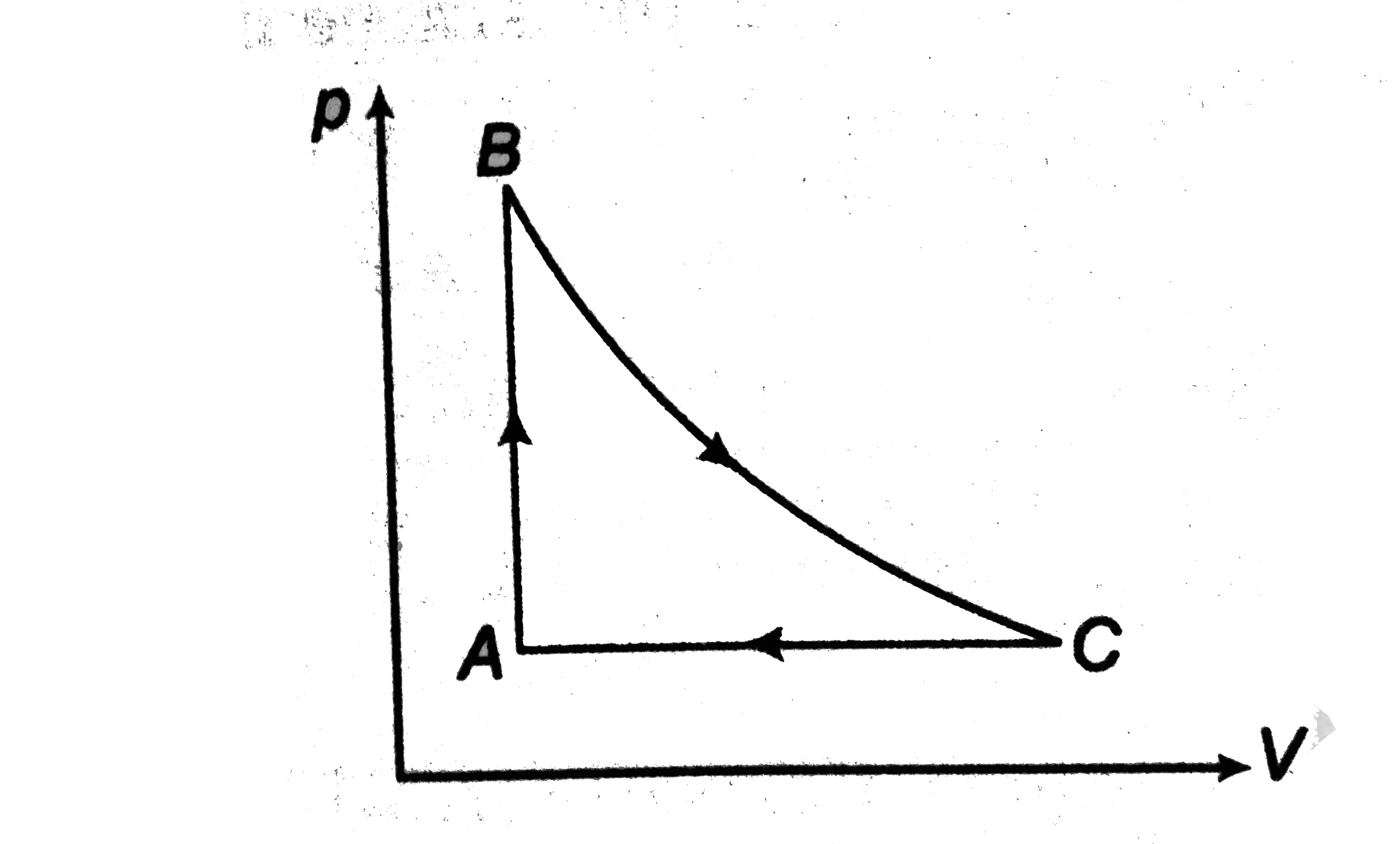
- One mole of a monoatomic ideal gas is taken through the cycle shown in...
Text Solution
|
- A thermodynamical process is shown in the figure with pA=3xxp(atm) , V...
Text Solution
|
- 1.0 k-mol of a sample of helium gas is put through the cycle of operat...
Text Solution
|
- In the figure, an ideal flows through the tube, which is of uniform cr...
Text Solution
|
- (a) A sample of 1.0 mol of a monoatomic ideal gas is taken through a ...
Text Solution
|
- One mole of a monatomic ideal gas is taken through the cycle shown in ...
Text Solution
|
- One mole of a monatomic ideal gas is taken through the cycle shown in ...
Text Solution
|