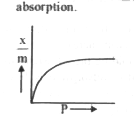
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
DISHA PUBLICATION|Exercise Exercise-2 :Concept Applicator|30 VideosSURFACE CHEMISTRY
DISHA PUBLICATION|Exercise Exercise-2 :Concept Applicator|30 VideosSTRUCTURE OF ATOM
DISHA PUBLICATION|Exercise Exercise|112 VideosTHE D- AND F- BLOCK ELEMENTS
DISHA PUBLICATION|Exercise EXERCISE - 2 CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-SURFACE CHEMISTRY-Exercise-1 : Concept Builder (TOPICEWISE)
- Which of the following statement is not correct
Text Solution
|
- The effect of pressure on adsorption is high if a. Temperature is lo...
Text Solution
|
- The heat evolved in chemisorption lies in the range (in kJ/mol) of :
Text Solution
|
- Blue colour of the sky is due to a. Adsorption of light by dust part...
Text Solution
|
- Which among the following statements is false ?
Text Solution
|
- In Langumir's model of adosrption of a gas on a solid surface :
Text Solution
|
- How many layers are adsorbed in chemical adsorption?
Text Solution
|
- Freundlich equation for adsorption of gases (in amount of Xg) on a sol...
Text Solution
|
- Which of the following statements is incorret about physisorption?
Text Solution
|
- The adsorption of gas on a solid surface varies with pressure of the g...
Text Solution
|
- Adsorption is the tendency of accumulation of molecular species at the...
Text Solution
|
- For adsorption of a gas on a solid, the plot of log (x//m) vs log P is...
Text Solution
|
- Which is adsorbed in maximum amount by activated charcoal?
Text Solution
|
- At high pressure, the entire surface gets covered by a monomolecular l...
Text Solution
|
- Adsorption is accompanied by :
Text Solution
|
- One gram of charcoal adsorbs 100 mL of 0.5 M CH(3)COOH to form a mono-...
Text Solution
|
- one gram of activated carbon has a surface are of 1000m^(2). Consideri...
Text Solution
|
- At 1 atm and 273 K the volume of nitrogen gas required to cover a samp...
Text Solution
|
- A sample of 16 g charcoal was brought into contact with CH(4) gas cont...
Text Solution
|
- What is shape - selective catalysis ?
Text Solution
|