Similar Questions
Explore conceptually related problems
Recommended Questions
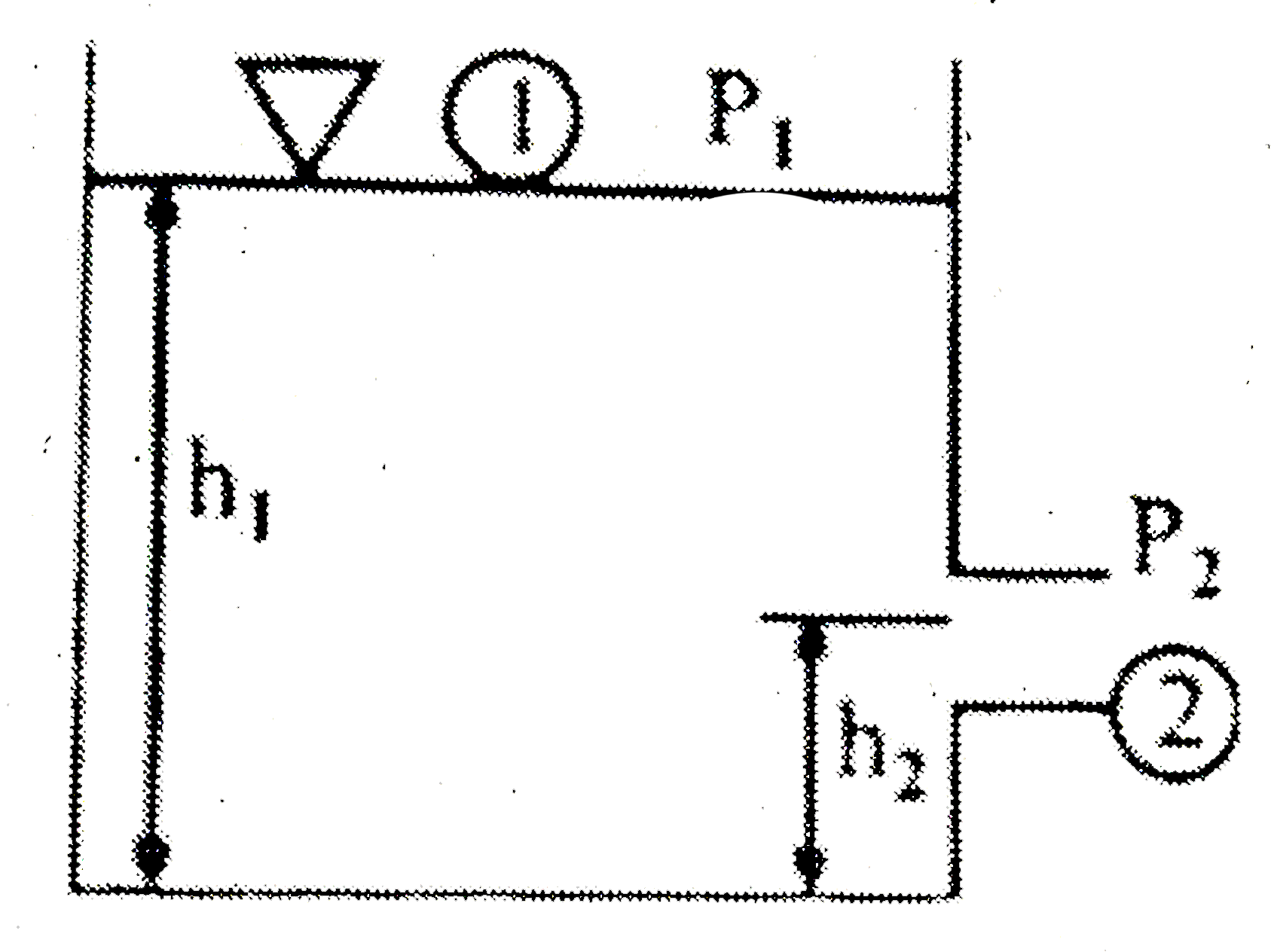
- using the illustration when both surfaces 1 and 2 are exposed to the a...
Text Solution
|
- m men and w women seat themselves at random on m + w seats arranged in...
Text Solution
|
- An ideal gas is initially at P1,V1 is expands to P2,V2 and then compre...
Text Solution
|
- using the illustration when both surfaces 1 and 2 are exposed to the a...
Text Solution
|
- using the illustration when both surfaces 1 and 2 are exposed to the a...
Text Solution
|
- using the illustration when both surfaces 1 and 2 are exposed to the a...
Text Solution
|
- At two points on a horizontal tube of varying cross-section, the radii...
Text Solution
|
- At two points on a horizontal tube of varying cross-section, the radii...
Text Solution
|
- Mr. A starts a business with an investment of Rs. 28,000. Mr. B joins ...
Text Solution
|