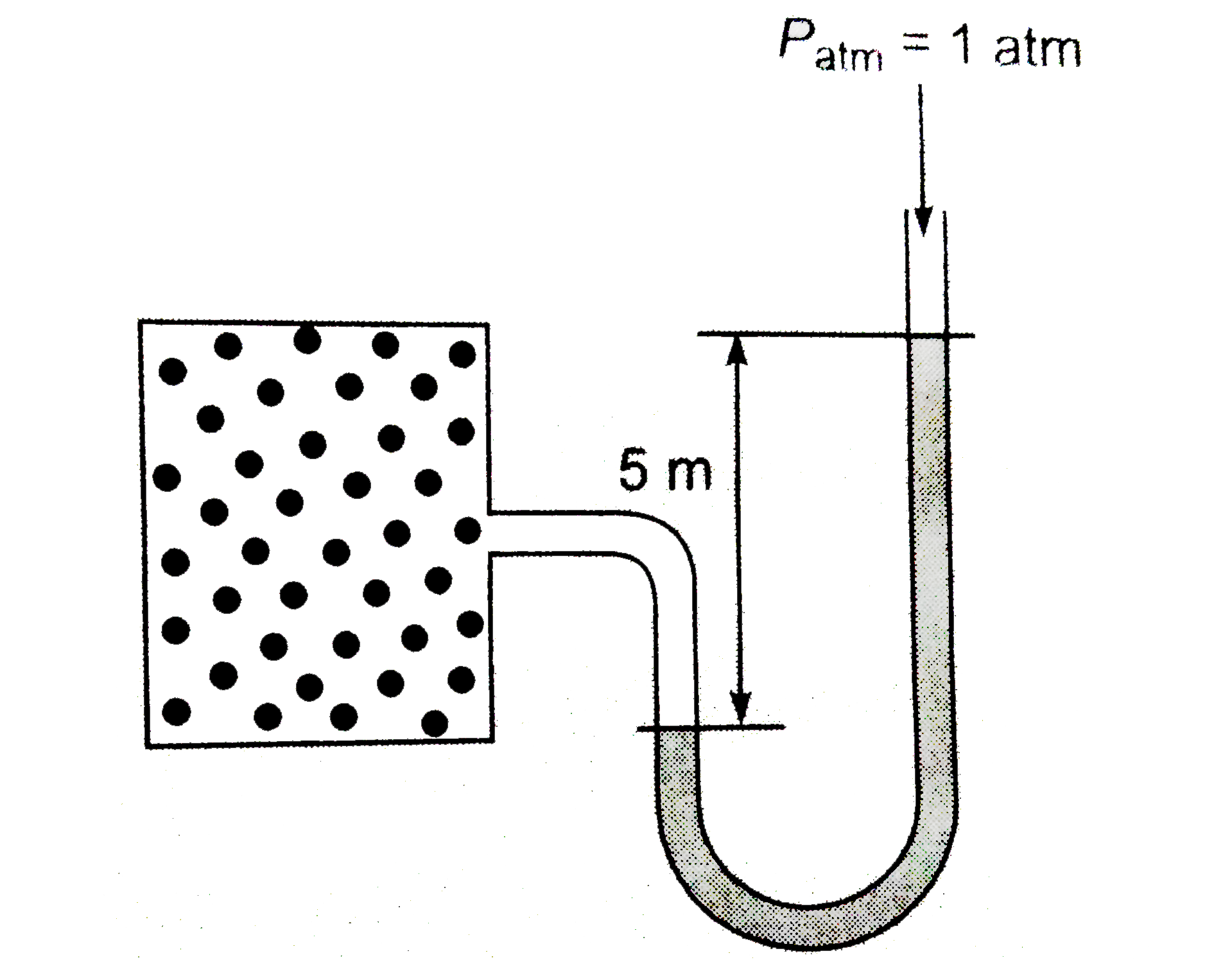Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the number of moles of gas present in the container of volum...
Text Solution
|
- The velocity of small ball of mass M and density ( d(1)= when dropped ...
Text Solution
|
- From the following set-up, calculate moles of the gas present in the c...
Text Solution
|
- If the gas in container gas pressure 77 cm of Hg, then calculate the h...
Text Solution
|
- 4 mL of a gas at 1 atm pressure and 300 K is dissolved in 1 L of solut...
Text Solution
|
- Calculate the number of moles of gas present in the container of volum...
Text Solution
|
- A gas jar of 10 litre volume filled with O(2) at 300 K is connected to...
Text Solution
|
- Calculate the number of moles of gas present in the container of volum...
Text Solution
|
- A 10-L vessel filled with O(2) at 30 K is connected to an open limb ma...
Text Solution
|