Similar Questions
Explore conceptually related problems
Recommended Questions
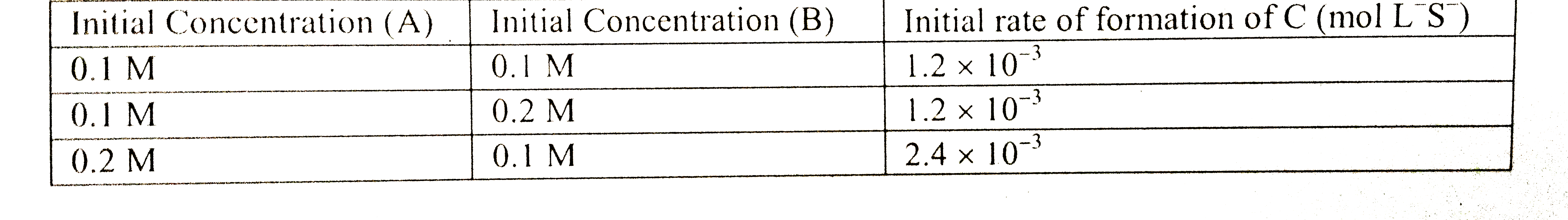
- For the non – stoichiometre reaction 2A + B rarr C + D , the following...
Text Solution
|
- For the non-stoichiometric reaction: 2A + B to C +D, the following k...
Text Solution
|
- The following initial rate data were obtained at 300K for the reaction...
Text Solution
|
- For the non – stoichiometre reaction 2A + B rarr C + D , the following...
Text Solution
|
- For the non-stoichiometre reaction 2 A + B to C + D , the following ki...
Text Solution
|
- 2A + B rarr C + D अभिक्रिया की बलगतिकी अध्ययन करने पर निम्नलिखित परिणा...
Text Solution
|
- For the non-stoichiometric reaction 2A +B rarr C+D, The following data...
Text Solution
|
- रासायनिक रिक्त अभिक्रिया में तीन पृथक् प्रयोगों में 298 K पर निम्न गति...
Text Solution
|
- रासायनिकता रिक्त अभिक्रिया 24 + BtoC+D में तीन पृथक् प्रयोगों में 298 ...
Text Solution
|